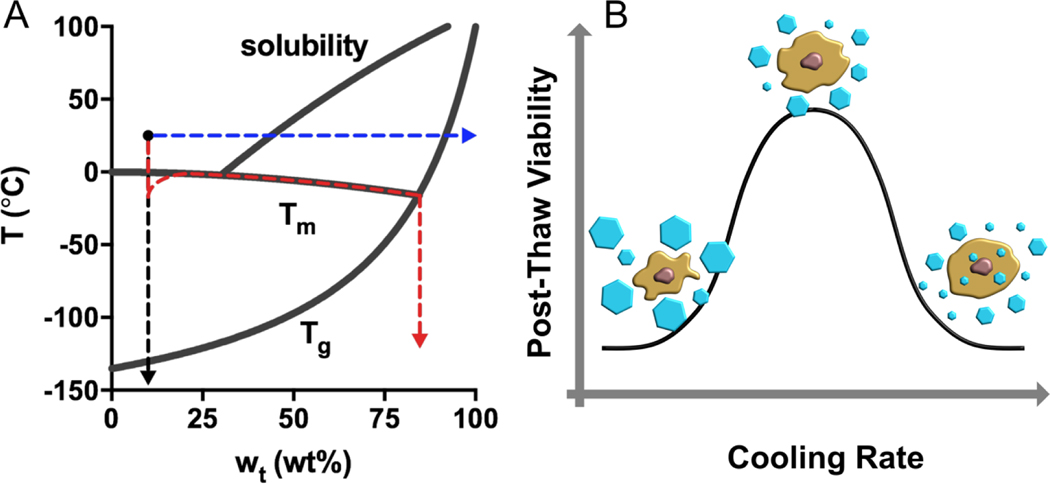
Figure 1.
Preservation pathways for a trehalose solution (initial condition: 10 wt% and 25 °C) and the two-factor hypothesis for slow-freezing of living cells. (A) In a slow-freezing method, ice crystallization drives the unfrozen trehalose solution to follow the liquidus curve until it crosses the (red). Direct vitrification strategy brings the temperature of the solution below ultra-rapidly in the absence of ice formation (black). Isothermal vitrification removes water from the solution until the concentration yields a that is below 25 °C (blue). (B) High cooling rates may be associated with intracellular ice formation that causes mechanical disruption of membranes and organelles; Slow cooling rates may result in excessive cell dehydration and prolonged exposure of cells to a high electrolyte concentration. There is an optimal cooling rate at which the two mechanisms of damage are balanced, yielding the highest post-thaw viability.