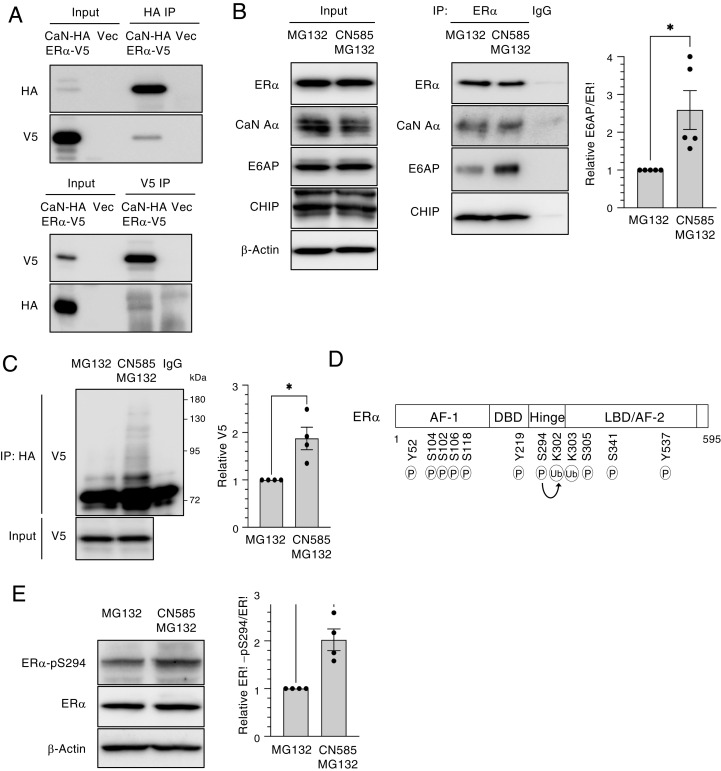
Fig. 2.
Calcineurin inhibits the ubiquitination (Ub) of ER-α by E6AP. (A) HEK293T cells were transiently transfected with expression vectors for HA-tagged calcineurin A–α (CaN-HA) and V5 epitope–tagged ER-α (ER-α–V5) or with the corresponding empty vector (Vec) for 2 d, after which total cell lysates were prepared and subjected to immunoprecipitation (IP) with antibodies to HA or to V5. The resulting immunoprecipitants as well as a portion of the original cell lysates (Input) were subjected to immunoblot analysis with the same antibodies. (B) MCF7 cells were treated with MG132 (10 μM) in the absence or presence of CN585 (30 μM) for 5 h, after which cell lysates were subjected to IP with antibodies to ER-α or with control immunoglobulin G (IgG). The resulting immunoprecipitants as well as a portion of the Input were subjected to immunoblot analysis with antibodies to ER-α, to calcineurin A–α, to E6AP, and to CHIP. The relative E6AP/ER-α band intensity ratio for the ER-α immunoprecipitants was determined as the mean ± SEM from five independent experiments. *P < 0.05 (paired t test). (C) HEK293T cells transiently transfected with expression vectors for HA-tagged ubiquitin (obtained from T. Ohta) and ER-α–V5 for 2 d were treated with MG132 in the absence or presence of CN585 for 6 h. Cell lysates were then prepared and subjected to IP with antibodies to HA or control IgG. The resulting immunoprecipitants as well as a portion of the Input were subjected to immunoblot analysis with antibodies to V5. The V5 band intensity was determined as the mean ± SEM from four independent experiments. *P < 0.05 (paired t test). (D) Functional domains of human ER-α. The transactivation domains (AF-1 and AF-2), DNA-binding domain (DBD), ligand-binding domain (LBD), and the hinge domain that links LBD and DBD are shown together with residues that are modified by phosphorylation (P) or Ub. The P of Ser294 has been associated with Ub. (E) MCF7 cells were treated with MG132 in the absence or presence of CN585 for 18 h, after which cell lysates were prepared and subjected to immunoblot analysis with antibodies to total or Ser294-phosphorylated (pS294) forms of ER-α. The relative phosphorylated/total ER-α band intensity ratio was determined as the mean ± SEM from four independent experiments. *P < 0.05 (paired t test).