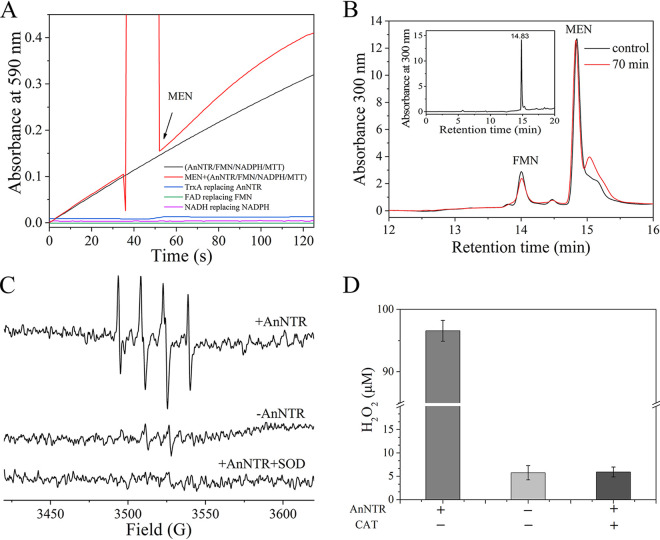
FIG 3.
AnNTR drives the one-electron metabolism of menadione, leading to ROS generation via redox cycling. (A) Reduction activity of recombinant AnNTR toward menadione. MTT was used as the ultimate electron acceptor of menadione reduction, and the MTT reduction product formazan was measured at 590 nm to measure the reduction due to menadione. The reaction mixture was 0.5 ml of sodium phosphate buffer (50 mM [pH 7.4]), NADPH (100 μM), DTPA (100 μM), FMN (10 μM), MTT (0.5 mM), and AnNTR (1.5 μg). The arrow indicates the time point of menadione (MEN; 50 μM) addition. As three controls, TrxA (2.6 μg) replacing AnNTR, NADH (100 μM) replacing NADPH, and FAD (10 μM) replacing FMN were added to the reaction solution in the presence of menadione. (B) No changes in menadione concentration were observed before or after menadione reduction catalyzed by AnNTR. After incubation for 70 min at 25°C, the reaction mixture was analyzed by using HPLC. The mixture without AnNTR was the control. (C) Confirmation of O2•− generation during menadione reduction procedure by EPR spectroscopy. DMPO was used as an O2•− trapper, and the four successive peaks are the characteristic spectrum of a DMPO–O2•− adduct. EPR spectra of the spin adduct of the reaction mixture obtained in the absence or presence of AnNTR or AnNTR plus SOD are shown. (D) H2O2 generation during the menadione reduction procedure. H2O2 was measured using hydrogen peroxide assay kits, and the absorbance was measured at 540 nm. Catalase (CAT) was employed to eliminate H2O2 in the reaction solution. The data are the means ± the SD of three independent experiments.