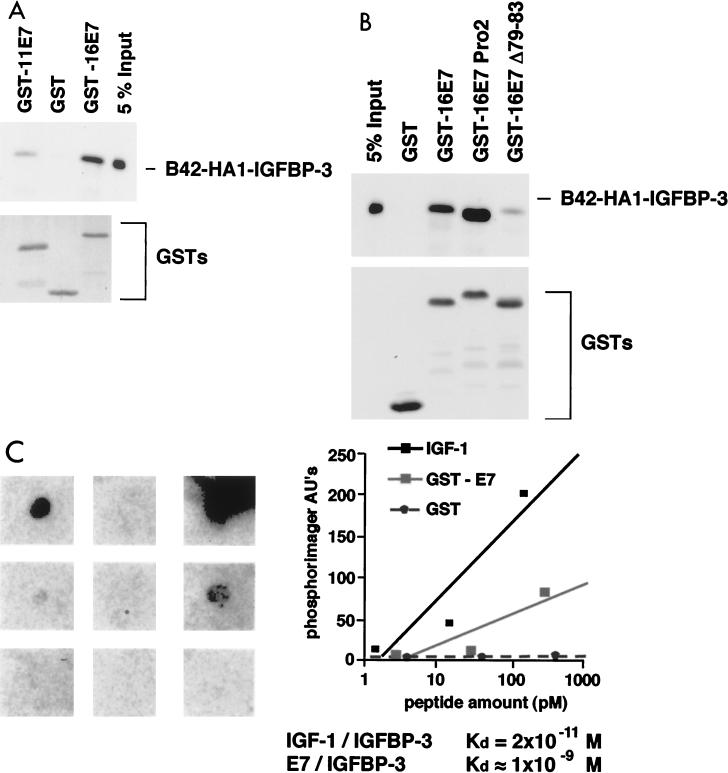
FIG. 2.
E7 binds IGFBP-3 in vitro. (A and B) Purified GST or GST-E7 fusion proteins immobilized on glutathione-Sepharose 4B beads were incubated with whole-cell extracts from yeast cells expressing a B42-HA1-IGFBP-3 fusion protein, as indicated. (Top) After washing, bound proteins were separated by gel electrophoresis and the amount of B42-HA1-IGFBP-3 protein that was retained was determined by direct immunoblotting using a monoclonal antibody against the HA1 epitope (94). For comparison, 5% of the input was also loaded on the gel. (Bottom) Input of the various GST fusion proteins was controlled by either Coomassie staining or Western blotting using anti-GST antibodies. (C) IGF-I and E7 proteins were immobilized on nitrocellulose filters as indicated and were probed with 125I-labeled IGFBP-3. The relative binding constants for both interactions are indicated. AU, arbitrary units.