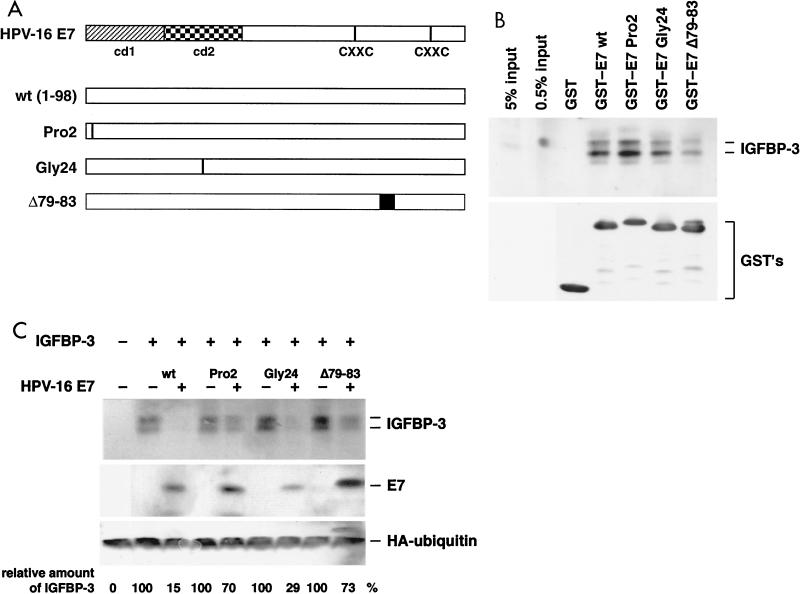
FIG. 6.
Analysis of E7 mutants for IGFBP-3 binding and degradation. (A) Structure of the HPV-16 E7 protein. cd1 and cd2 and the putative zinc finger motifs (CXXC) (81) are indicated. The mutants of E7 used in the assays are shown. (B) IGFBP-3 from transiently transfected U-2OS cells was incubated with glutathione- Sepharose 4B beads containing various GST-E7 fusion proteins as indicated. (Top) After elution from the beads, bound proteins were separated by gel electrophoresis and IGFBP-3 was detected by Western blotting; for comparison, samples with 0.5 and 5% of the IGFBP-3 input were also loaded on the gel. (Bottom) Input of the various GST fusion proteins was controlled by anti-GST immunoblotting. (C) U-2OS cells were transfected with expression vectors for IGFBP-3, wild-type E7, and E7 mutants, as indicated. The levels of IGFBP-3 and E7 proteins were determined by Western blotting. Relative IGFBP-3 protein levels are given. Values are the means of three independent experiments. To control transfection efficiency, HA-tagged ubiquitin was cotransfected and detected by Western blotting.