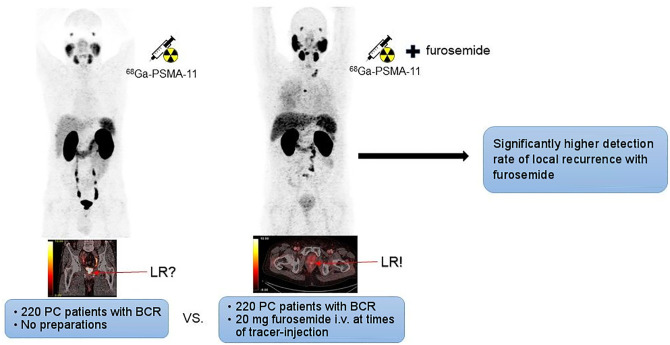
Visual Abstract
Keywords: prostate cancer, PET, PET/CT, prostate specific membrane antigen, 68Ga-PSMA-11, furosemide, forced diuresis, biochemical recurrence, local recurrence
Abstract
The aim of this study was twofold. First, we aimed to assess the impact of forced diuresis with early furosemide injection on the detection rate of local recurrence in prostate cancer patients with biochemical recurrence referred for 68Ga-labeled Glu-NH-CO-NH-Lys(Ahx)-HBED-CC (68Ga-PSMA-11) PET/CT. Second, we determined whether intravenous administration of furosemide shortly after tracer injection increases renal washout of 68Ga-PSMA-11 before it binds to the PSMA receptor with possible influence on biodistribution and intensity of tracer uptake in organs with physiologic tracer accumulation. Methods: In a retrospective analysis, 2 different groups with 220 prostate cancer patients each, referred for 68Ga-PSMA-11 PET/CT because of biochemical recurrence after primary therapy, were compared: patients in group 1 (median prostate-specific antigen, 1.30 ng/mL) received no preparation before imaging, whereas patients in group 2 (median prostate-specific antigen, 0.82 ng/mL) were injected with 20 mg of furosemide and 500 mL of sodium chloride (NaCl 0.9%) immediately after tracer injection. The presence of local recurrence was assessed visually. In addition, the intensity of tracer accumulation in organs with physiologic tracer uptake was evaluated. Results: The detection rate of lesions judged positive for local recurrence was significantly higher in patients receiving furosemide than in patients without preparation: 56 cases (25.5%) versus 38 cases (17.3%), respectively (P = 0.048). Median maximum SUVs (SUVmax) of organs with physiologic uptake of 68Ga-PSMA-11 in groups 1 and 2 were urinary bladder (63.0 vs. 8.9), kidney (55.6 vs. 54.5), liver (9.9 vs. 9.4), spleen (11.2 vs. 11.9), small bowel (16.2 vs. 17.1), parotid gland (19.2 vs. 19.6), lacrimal gland (8.9 vs. 10.9), blood-pool activity (2.2 vs. 2.3), muscle (1.0 vs. 1.1), and bone (1.6 vs. 1.6). Apart from bladder activity, no significant reduction of tracer accumulation was found in the patient group receiving furosemide. Conclusion: Injection of 20 mg of furosemide at the time point of radiotracer administration significantly increases the detection rate of local recurrence in prostate cancer patients with biochemical recurrence referred for 68Ga-PSMA-11 PET/CT. As intensity of 68Ga-PSMA-11 uptake in organs with physiologic uptake is not significantly reduced, a negative impact of early furosemide injection on targeting properties and biodistribution of 68Ga-PSMA-11 seems unlikely.
PET/CT with radiotracers binding to the prostate specific membrane antigen (PSMA) has become a clinically accepted imaging method in prostate cancer (PC) patients presenting with biochemical recurrence (BR) after primary therapy (1–5). Several PSMA-ligands currently are used for PET imaging (6), of which 68Ga-labeled Glu-NH-CO-NH-Lys(Ahx)-HBED-CC (68Ga-PSMA-11), an inhibitor of the PSMA receptor, represents one of the most frequently used PSMA tracers worldwide (7). Usually, 68Ga-PSMA-11 PET/CT is acquired 60 min after injection (3). However, at this time point physiologically high tracer uptake is present in the urinary bladder, which often causes difficulties in the evaluation of anatomic structures surrounding the urinary bladder (8–10). In particular, local recurrence (LR) may be missed in PC patients with BR (11). In this context, PSMA tracers with lower physiologic accumulation in the urinary bladder (e.g., 18F-PSMA-1007) may be advantageous (12).
In 68Ga-PSMA-11 PET/CT, several techniques can be used to enhance diagnostic certainty in regions adjacent to the urinary bladder. One approach is to perform imaging at a second time point very early after injection of 68Ga-PSMA-11, when tracer accumulation in the urinary bladder is still absent or low (13). Early dynamic imaging starting simultaneously with tracer injection or early static PET examinations performed shortly after injection of the tracer have been proven to be helpful in distinguishing malignant PC lesions from urinary bladder activity (13,14).
Administration of diuretics is another strategy to decrease urinary bladder activity in 68Ga-PSMA-11 PET/CT. In fact, administration of 20 mg of furosemide combined with oral hydration of 500 mL of water is recommended in the joint European Association of Nuclear Medicine (EANM) and Society of Nuclear Medicine and Molecular Imaging (SNMMI) procedure guideline for PSMA-ligand PET/CT (6). It has been shown that tracer accumulation in the urinary bladder of 68Ga-PSMA-11 but also of 68Ga-PSMA I&T, another PSMA-ligand with predominantly renal excretion, can be reduced with furosemide on PET scans 60 min after injection, but also on late examinations with image acquisition 90 and 180 min after injection (15–18). In a recent study by our group, we demonstrated that intravenous injection of 20 mg of furosemide at the time of radiotracer injection followed by an infusion of 500 mL of sodium chloride significantly reduced urinary bladder activity (19). However, to date no data of a large patient cohort are available on whether reduction of urinary bladder activity achieved by an early furosemide injection also results in a higher detection rate of LR in PC patients with BR. In addition, there are concerns that injection of furosemide shortly after tracer administration may cause a relevant renal washout of 68Ga-PSMA-11 before it binds to the PSMA receptor. Therefore, in the present study we investigated whether forced diuresis with early furosemide injection has a positive impact on the detection rate of LR in comparison with patients receiving no preparation and whether administration of furosemide simultaneously with tracer injection has a negative influence on intensity of tracer accumulation in organs with physiologic tracer uptake.
MATERIALS AND METHODS
Patient Population
For this retrospective analysis, a total of 440 PC patients who were referred to 68Ga-PSMA-11 PET/CT between January 11, 2016, and September 25, 2018, for assessment of BR after definitive primary therapy were extracted from our database. Search for patients in our archive centered on June 26, 2017, when administration of 20 mg of furosemide and hydration was introduced as standard protocol in our clinical routine, following the EANM/SNMMI guideline on PSMA PET/CT (6). The analysis also comprised PET/CT examinations of patients partly included in previous publications by our group (13,19). In group 1 (G1), 220 consecutive scans of patients who were investigated before June 26, 2017, were included. Group 2 (G2) comprised 220 consecutive scans of patients examined after June 26, 2017, who were injected with 20 mg of furosemide at the time of tracer administration, followed by intravenous hydration with 500 mL of sodium chloride (NaCl 0.9%). Patient characteristics of the 2 groups are presented in Tables 1 and 2. The study concept was presented to our institutional ethics committee. As the study was designed retrospectively, using data obtained for clinical purposes, formal ethical approval was not deemed necessary by the ethics committee, meeting the legal requirements of our country. Written informed consent was obtained from all patients before the examination. All procedures performed in this study were in accordance with the principles of the 1964 Declaration of Helsinki and its subsequent amendments (20)
TABLE 1.
Patient Characteristics and Summary of Previous Treatment in the 2 Different Patient Preparation Groups
| Group | Median age (y) | Median PSA (ng/mL) at PET | Median PSAdt (mo) | Median BMI kg/m2 | GFR < 60 mL/min/1.73m2 | ADT at PET | BR after RPE | BR after pRt | BR after RPE + sRT |
|---|---|---|---|---|---|---|---|---|---|
| G1* | 70 (range, 52–87) | 1.30 (range, 0.14–81.09) | 6 (range, 0.9–120.2)† | 25.9 (range, 17.5–57.8) | 38 (17.3%) | 40 (18.2%) | 109 (49.5%) | 22 (10.0%) | 89 (40.5%) |
| G2‡ | 72 (range, 44–88) | 0.82 (range, 0.10–147.6) | 4.7 (range, 1.2–59.1)¶ | 26.0 (range, 18.8–40.8) | 34 (15.5%) | 46 (20.9%) | 116 (52.7%) | 40 (18.2%) | 64 (29.1%) |
| P | 0.032 | 0.055 | 0.417 | 0.613 | 0.515 | 0.809 | § | § | § |
No preparation.
220 patients, missing data n = 171.
Injection of 20 mg of furosemide and 500 mL of NaCl 0.9%.
220 patients, missing data n = 172.
Overall P value 0.009.
P values from a Mann–Whitney U test (age, PSA, PSAdt, BMI) and from Fisher exact tests (GFR, ADT, and BR subgroups).
ADT = androgen deprivation therapy; BMI = body mass index; GFR = glomerular filtration rate; PSAdt = PSA doubling time; pRT = primary radiation therapy; sRT = salvage radiation therapy.
TABLE 2.
Tumor Characteristics in the 2 Different Patient Preparation Groups
| Gleason score* | T stage† | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | GS 5 | GS 6 | GS 7a | GS 7b | GS 8 | GS 9 | GS 10 | T1 | T2 | T3 | T4 |
| G1‡ (%) | 0 (0.0%) | 17 (8.9%) | 62 (32.5%) | 35 (18.3%) | 30 (15.7%) | 47 (24.6%) | 0 (0.0%) | 2 (1.3%) | 57 (36.1%) | 85 (53.8%) | 14 (8.9%) |
| G2¶ (%) | 2 (1.0%) | 17 (18.1%) | 76 (36.2%) | 45 (21.4%) | 30 (14.3%) | 39 (18.6%) | 1 (0.5%) | 4 (2.4%) | 63 (37.3%) | 100 (59.2%) | 2 (1.2%) |
Missing data (G1 n = 29; G2 n = 10).
Missing data (G1 n = 62; G2 n = 51).
No preparation.
Injection of 20 mg of furosemide and 500 mL of NaCl 0.9%.
P value from Fisher exact tests. Overall P value for Gleason score was 0.531, and overall P value for T stage was 0.008.
Radiopharmaceutical
PSMA-11 (Glu-NH-CO-NH-Lys(Ahx)-HBED-CC; HBED = N,N'-bis[2-hydroxy-5-(carboxyethyl)benzyl]ethylenediamine-N, N'-diacetic acid) was obtained from ABX Advanced Biochemical Compounds in good-manufacturing-practice quality. 68Ga-PSMA-11 was prepared on an automated synthesis module (Modular-Lab PharmTracer; Eckert & Ziegler) using a procedure previously described (14,21). The radiochemical purity of the final product was >92% as analyzed by reversed-phase high-performance liquid chromatography analysis.
Imaging Protocol
68Ga-PSMA-11 PET/CT imaging was conducted using a dedicated PET/CT system in time-of-flight mode (Discovery 690; GE Healthcare). Patients received a median activity of 154.8 MBq (range: 95.0–216.0 MBq). The median injected activity of 68Ga-PSMA-11 did not differ significantly between patients of G1 and G2 (150.5 MBq vs 156.4 MBq; P = 0.605). The median uptake time in G1 and G2 was 67 min (range: 52–101; Q1 = 60 min; Q3 = 73 min) and 69 min (range: 45–100; Q1 = 61 min; Q3 = 77 min), respectively, and differed significantly (P= 0.022). A whole-body PET scan (skull vertex to upper thighs) in 3-dimensional mode was acquired (emission time: 2 min per bed position with an axial field of view of 15.6 cm per bed position). Two hundred twenty-five patients (51.1%) received a diagnostic contrast-enhanced CT scan. The contrast-enhanced CT scan parameters using GE smart mA dose modulation were 100 or 120 kVp; 80–450 mA; noise index, 24; 0.8 s per tube rotation; slice thickness, 3.75 mm; and pitch, 0.984. A CT scan of the thorax, abdomen, and pelvis (shallow breathing) was acquired 40–70 s after injection of contrast agent (60–120 mL of Iomeron 400 mg/L, depending on patient body weight), followed by a CT scan of the thorax in deep inhalation. In the remaining 215 patients (48.9%), a low-dose CT scan was acquired for attenuation correction of the PET emission data. Low-dose CT was also used for anatomic allocation of lesions with increased uptake found on PET. The low-dose CT scan parameters using GE smart mA dose modulation were 100 kVp; 15–150 mA; noise index, 60; 0.8 s per tube rotation; slice thickness, 3.75 mm; and pitch, 1.375. Reconstruction was performed with an ordered-subset expectation maximization algorithm with 4 iterations/8 subsets. Images were corrected for randoms and scatter.
Image Analysis
All 68Ga-PSMA-11 PET/CT images were analyzed with dedicated commercially available software (Advance Workstation SW, version AW4.5 02; GE Healthcare), which allowed the review of PET, CT, and fused imaging data in axial, coronal, and sagittal slices. PET images were interpreted independently by 2 board-approved nuclear medicine physicians, who were masked to the method of patient preparation, clinical patient data, and results of other examinations. In the case of patients already included in previous studies, readers were not aware of results of those analyses. If an early static PET scan was performed (13), only images 60 min after injection were assessed. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) diagnostic criteria for 68Ga-PSMA-11 PET/CT reporting in PC proposed by Eiber et al. (22) and consensus criteria for image interpretation defined by Fanti et al. served as a reference for assessment of LR (8). Patients were judged either positive or negative for LR. Cases in which a clear distinction between urinary activity and a LR was not possible were classified as equivocal. In the case of disagreement between the 2 readers, images were reevaluated and a final diagnosis was reached in consensus. In addition, intensity of tracer uptake in organs and tissues with physiologic tracer accumulation and lesions judged as LR was measured, using maximum and mean SUV (SUVmax and SUVmean, respectively). For SUV calculations, volumes of interest were generated automatically by the software (Advance Workstation SW, version AW4.5 02; GE Healthcare) with a manually adapted isocontour threshold centered on the organs of interest. Tracer uptake was measured in the following organs and tissues: urinary bladder, kidneys, small bowel, liver, spleen, parotid gland, lacrimal gland, blood pool (aortic arch), muscle activity (gluteal region), and bone (thoracic vertebra).
Statistical Analysis
Differences between the 2 groups (G1: patients without preparation vs. G2: patients receiving furosemide) were evaluated using nonparametric testing procedures. Distributions of continuous variables (baseline characteristics and SUVmax in various tissues) were compared between groups using Mann–Whitney U tests. LR detection rates (positive, negative, equivocal) were compared between groups using Fisher exact tests. All tests of statistical significance were 2-sided, and P values of less than 0.05 were considered statistically significant. All analyses were conducted in R, version 3.5.1 (The R Foundation) (23).
RESULTS
In brief, comparison of patient characteristics between both preparation groups revealed a statistically significant difference in patient age, method of definitive treatment before the PET examination, and T-stage, whereas other parameters listed in detail in Tables 1 and 2 did not differ significantly.
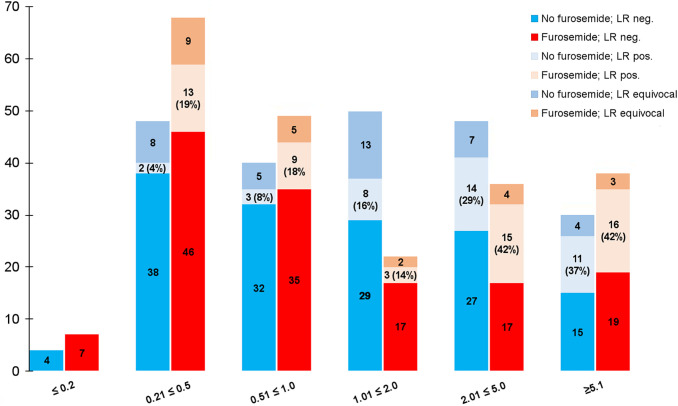
With regard to visual evaluation of LR, a statistically significantly higher number of LR could be detected in the patient group that received furosemide compared with patients without preparation, with 56 cases (25.5%) and 38 cases (17.3%) judged positive for LR, respectively (P = 0.048). Frequency of equivocal findings in the prostatic fossa was markedly lower in the group with furosemide in comparison with patients without preparation, with 27 (12.3%) versus 37 (16.8%) unclear cases. However, the difference between both groups in this respect did not reach statistical significance (P = 0.223). Intensity of tracer uptake in lesions considered as LR did not differ significantly between both groups (P = 0.987), showing a median SUVmax of 8.1 in G1 (range: 3.2–47.9) and 8.4 in G2 (range: 3.2–139.0). An overview of these results is also shown in Table 3, where a subgroup analysis of patients according to primary treatment is presented as well. In addition, prostate-specific antigen (PSA) subgroups were analyzed and compared with respect to detection of LR (demonstrated in Fig. 1).
TABLE 3.
Results of Visual Assessment of LR for Main Groups and Primary Therapy Subgroups
| Group | G1* | G2† | P |
|---|---|---|---|
| Overall, LR positive (n) | 38 (17.3%) | 56 (25.5%) | 0.048 |
| Overall, LR negative (n) | 145 (65.9%) | 137 (62.3%) | 0.487 |
| Overall, LR equivocal (n) | 37 (16.8%) | 27 (12.3%) | 0.223 |
| Median SUVmax, LR positive (n) | 8.1 (range, 3.2–47.9) | 8.4 (range, 3.2–139.0) | 0.987 |
| Subgroup pRT, LR positive (n) | 12 (54.5%) | 20 (50.0%) | 0.795 |
| Subgroup pRT, LR negative (n) | 8 (36.4%) | 15 (37.5%) | 1 |
| Subgroup pRT, LR equivocal (n) | 2 (9.1%) | 5 (12.5%) | 1 |
| Subgroup pRPE, LR positive (n) | 26 (13.1%) | 36 (20.0%) | 0.095 |
| Subgroup pRPE, LR negative (n) | 137 (69.2%) | 126 (70.0%) | 0.911 |
| Subgroup pRPE, LR equivocal (n) | 35 (17.7%) | 18.0 (10.0%) | 0.038 |
No preparation.
Injection of 20 mg of furosemide and 500 mL of NaCl 0.9%.
P values from Fisher exact tests for frequencies and from Mann–Whitney U test for median SUVmax comparison.
pRPE = primary radical prostatectomy; pRT = primary radiation therapy; SUVmax = maximal SUV.
FIGURE 1.
Comparison of number of patients judged positive (pos.), negative (neg.), or equivocal for LR in relation to PSA level, together with PET-positive rate of LRs in each subgroup (%). G1 (no furosemide, blue column); G2 (with furosemide, red column).
Intensity of tracer accumulation in the urinary bladder was significantly higher in patients without preparation than in the furosemide group, showing a median SUVmax of 63.0 and 8.9, respectively (P < 0.001). Regarding intensity of physiologic tracer uptake in the remaining predefined organs and tissues, no statistically significant difference in median SUVmax could be found between the 2 groups in the kidneys (55.6 vs. 54.5), the liver (9.9 vs. 9.4), the spleen (11.2 vs. 11.9), the parotid gland (19.2 vs. 19.8), the blood pool (2.2 vs. 2.3), and the bone (1.6 vs. 1.6). Higher SUVmax in the furosemide group in comparison with G1, reaching statistical significance, was measured in the small bowel (17.1 vs. 16.2; P = 0.040), the lacrimal gland (10.9 vs. 8.9; P < 0.001), and the muscle (1.1 vs. 1.0; P = 0.001). In total, intensity of physiologic tracer uptake in all organs and tissues investigated (except the urinary bladder) was not reduced significantly in the patient group receiving furosemide compared with the patient group without preparation. A detailed synopsis of SUVs including SUVmean is given in Tables 4 and 5.
TABLE 4.
Comparison of Intensity of Tracer Accumulation in Organs and Tissues with Physiologic Tracer Uptake
| Point of measurement | G1* | G2† | P |
|---|---|---|---|
| SUVmax median bladder | 63.0 (4.6–350.0) | 8.9 (1.7–35.0) | <0.001 |
| SUVmax median kidneys | 55.6 (7.4–100.9) | 54.5 (16.1–94.6) | 0.264 |
| SUVmax median liver | 9.9 (4.5–43.4) | 9.4 (3.0–19.5) | 0.196 |
| SUVmax median spleen | 11.2 (4.2–34.0) | 11.9 (3.5–23.4) | 0.558 |
| SUVmax median small bowel | 16.2 (4.7–36.9) | 17.1 (8.3–51.3) | 0.040 |
| SUVmax median parotid gland | 19.2 (9.1–39.3) | 19.8 (10.7–36.0) | 0.069 |
| SUVmax median lacrimal gland | 8.9 (3.0–23.9) | 10.9 (2.8–31.9) | <0.001 |
| SUVmax median blood pool | 2.2 (1.2–4.6) | 2.3 (1.2–4.9) | 0.649 |
| SUVmax median muscle | 1.0 (0.5–3.0) | 1.1 (0.6–2.2) | 0.001 |
| SUVmax median bone | 1.6 (0.8–3.8) | 1.6 (0.8–4.3) | 0.564 |
No preparation.
Injection of 20 mg of furosemide and 500 mL of NaCl 0.9%.
P values from Mann–Whitney U tests. Data in parentheses are ranges.
SUVmax = maximal SUV.
TABLE 5.
Comparison of Intensity of Tracer Accumulation in Organs and Tissues with Physiologic Tracer Uptake
| Point of measurement | G1* | G2† | P |
|---|---|---|---|
| SUVmean median bladder | 41.9 (22.8–69.8) | 5.3 (3.5–7.3) | <0.001 |
| SUVmean median kidneys | 34.8 (29.8–40.6) | 34.1 (27.8–40.4) | 0.358 |
| SUVmean median liver | 5.4 (4.4–6.5) | 5.2 (4.4–6.6) | 0.679 |
| SUVmean median spleen | 6.5 (5.2–8.5) | 7.0 (5.8–8.3) | 0.070 |
| SUVmean median small bowel | 9.4 (7.5–11.6) | 10.0 (8.23–12.0) | 0.027 |
| SUVmean median parotid gland | 12.0 (10.2–13.9) | 12.4 (10.9–15.0) | 0.028 |
| SUVmean median lacrimal gland | 5.8 (4.4–7.2) | 7.0 (5.4–9.0) | <0.001 |
| SUVmean median blood pool | 1.3 (1.1–1.5) | 1.3 (1.1–1.5) | 0.990 |
| SUVmean median muscle | 0.6 (0.5–0.7) | 0.6 (0.5–0.7) | 0.011 |
| SUVmean median bone | 0.9 (0.8–1.1) | 0.9 (0.7–1.1) | 0.560 |
No preparation.
Injection of 20 mg of furosemide and 500 mL of NaCl 0.9%.
P values from Mann–Whitney U tests. Data in parentheses are ranges.
SUVmean = mean SUV.
Histologic confirmation of malignant origin of cases judged positive for LR could not be achieved. However, LR could be verified in 50.0% of cases in G1 (n = 19) and 42.9% of cases in G2 (n = 24). LR was confirmed either radiologically with a pathologic correlate on diagnostic CT, MRI, or transrectal ultrasound (n = 33) or on a follow-up 68Ga-PSMA-11 PET/CT scan (n = 4), or patients showed a decrease of PSA values after salvage radiation therapy of the prostatic fossa after PSMA-11 PET/CT (n = 6).
In a subgroup analysis of all patients rated positive for LR on 68Ga-PSMA-11 PET/CT (G1: n = 38; G2: n = 56), LR was the only site of recurrence in 24 cases (63.1%) in G1 and in 31 cases (55.4%) in G2. In 8 cases in G1 (21.1%) and 13 cases in G2 (23.2%), PSMA-positive lymph nodes classified as metastases could be detected in addition to LR without sign of metastases to bone or other organs. Two cases in G1 (5.3%) and 6 cases in G2 (10.7%) showed an LR and PSMA-positive metastases both to lymph nodes and in the bone. LR and PSMA-positive skeletal metastases without PSMA-positive lymph nodes were present in 4 cases in G1 (10.5%) and 6 cases in G2 (10.7%).
Overall, detection rate of at least 1 PSMA-positive lesion consistent with recurrent PC was 60.5% of all patients in G1 (n = 133) and 66.8% of all patients in G2 (n = 147), not differing significantly (P = 0.198). In G1, PSMA-positive lymph node metastases were detected in 81 cases (36.8%), PSMA-avid skeletal metastases in 34 cases (15.5%), and hematogenous metastases other than bone in 4 cases (1.8%). In G2, PSMA-positive lymph node metastases were found in 91 cases (41.4%), whereas bone metastases and nonskeletal distant metastases were present in 36 cases (16.4%) and 5 cases (2.3%), respectively.
Patients were also asked whether they felt urinary urgency during the examination, categorizing it as slight, moderate (tolerable), or strong (major discomfort). In G1, only 9 patients (4.1%) stated urgency, which was described as slight to moderate. In the furosemide group, urinary urge during scanning occurred in 93 cases (42.3%) and was classified by the patients in most cases as slight (n = 77; 82.8%) and moderate (n = 16; 17.2%). In total, early injection of 20 mg of furosemide was tolerated well, no strong urinary urge during the PET examination was reported, and no furosemide-induced adverse reaction was recorded.
DISCUSSION
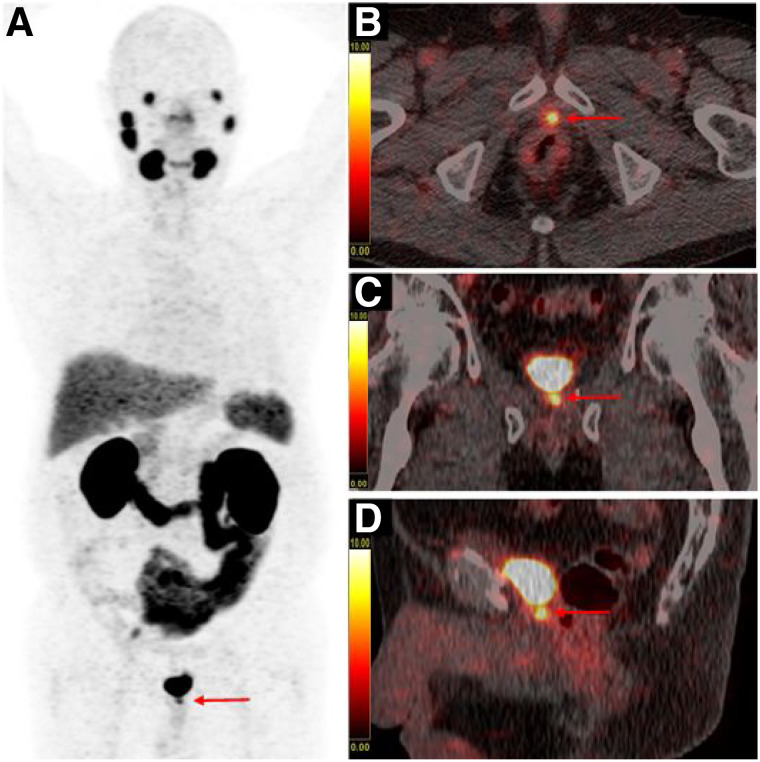
In 68Ga-PSMA-11 PET/CT, usually conducted 60 min after tracer injection, LR may be overlooked when it is located adjacent to the urinary bladder, mainly due to the masking effect of overlaying urinary bladder activity (11). In addition, there are cases in which it is almost impossible to discriminate LR from urinary activity within the urethra or the bladder neck, especially at the site of anastomosis after RP (8), as displayed in an example in Figure 2.
FIGURE 2.
Example of an equivocal finding in prostate fossa on 68Ga-PSMA-11 PET/CT with maximum-intensity-projection (A), fused axial (B), fused coronal (C), and fused sagittal (D) images of a PC patient with BR after radical prostatectomy and salvage radiation therapy (PSA: 1.34 ng/mL), receiving no preparation before imaging. Intense focal uptake is present in midline at level of vesicourethral anastomosis (red arrow). Clear distinction between LR and urinary activity within urethra is not possible (SUVmax of focal uptake in midline: 10.3 and SUVmax of urinary bladder: 118.0).
Administration of furosemide is recommended in the joint EANM/SNMMI procedure guideline for 68Ga-PSMA-11 PET/CT (6). In a recently published study by our group, we demonstrated that forced diuresis with 20 mg of furosemide, injected simultaneously with the radiotracer, significantly reduced tracer accumulation in the urinary bladder compared with patients receiving no preparation or hydration alone (19).
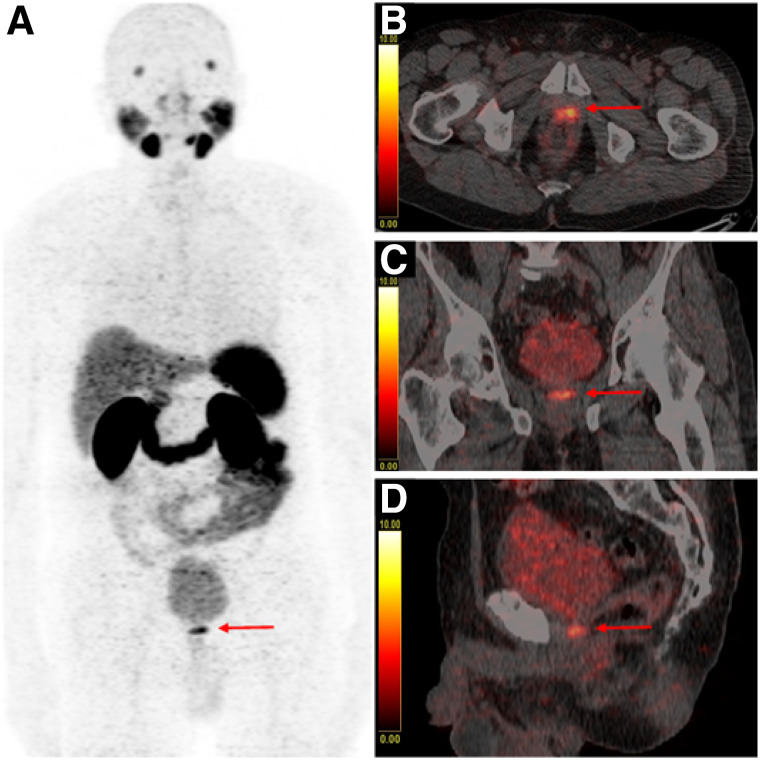
The primary objective of the present study was to investigate whether the afore-described furosemide-induced reduction of urinary bladder activity also improved detection rates of LR and enhanced diagnostic certainty in the prostate fossa. Indeed, our study revealed a significantly higher number of PSMA-positive LRs in the furosemide group than in the group of patients without preparation, with 56 and 38 cases judged positive for LR, respectively (25.5% vs. 17.3% of patients). The effect of furosemide-induced tracer washout of the urinary bladder on detectability of LR is illustrated in Figure 3. Furthermore, the number of equivocal findings in the prostate fossa was lower in patients undergoing forced diuresis in comparison with patients without furosemide, with 27 and 37 unclear cases, respectively (12.3% vs. 16.8% of patients). However, the difference in this respect did not reach statistical significance. This may be attributable to the fact that tracer activity does not vanish completely from the urinary routes after furosemide administration, and in a small number of cases a relatively high amount of tracer remains within the bladder and urethra despite forced diuresis (15,21,24). Of note, our data suggest that patients who have undergone radical prostatectomy (RPE) profit most from furosemide. The number of unclear findings after RPE was significantly lower in the furosemide group and a clear tendency in the positivity rate of LR was found after furosemide, although these did not reach statistical significance. In contrast, the detection rate of LR in both groups was almost the same in patients who underwent primary radiation therapy without RPE (as presented in Table 3). Our results are in line with the findings of a study conducted by Fennessy et al., using a similar preparation protocol with early administration of furosemide (15). Fennessy et al. reported that diagnostic confidence in the pelvic area on 68Ga-PSMA-11 PET/CT could be improved in patients injected with furosemide. However, the number of patients in the patient subset with BR was relatively low in this analysis (n = 44) and the analysis was probably underpowered to detect a positive effect of the furosemide protocol on detection rates of LR.
FIGURE 3.
68Ga-PSMA-11 PET/CT with maximum-intensity-projection (A), fused axial (B), fused coronal (C), and fused sagittal (D) images of a PC patient with BR after radical prostatectomy (PSA: 1.18 ng/mL), who received forced diuresis with 20 mg of furosemide simultaneously with radiotracer injection. Focal uptake of high intensity with SUVmax of 9.6 is present in area of vesicourethral anastomosis (red arrow) that can be clearly distinguished from adjacent urinary activity in bladder (SUVmax of 9.4), representing case consistent with LR. Malignant origin of finding was confirmed on subsequent MRI.
There are concerns whether injection of furosemide at the time of radiotracer injection is appropriate. Injection of furosemide early in the uptake phase of the tracer might lead to an increased renal washout of 68Ga-PSMA-11 before it binds to PSMA, possibly resulting in a reduced tracer uptake in tumor lesions and in lower sensitivity of the examination. We tried to address this issue by comparing intensity of tracer accumulation in organs and tissues with physiologic 68Ga-PSMA-11 uptake in patients receiving furosemide and patients without preparation. We hypothesized that if a significant increase of renal tracer excretion was induced by early injection of furosemide, it would result in a lower tracer uptake in organs with physiologic uptake. However, our results clearly showed that, apart from the urinary bladder, tracer accumulation in all organs and tissues investigated was not lower in the patient group receiving furosemide than in the patient group without preparation. Calculated SUVs are comparable to those of previous studies dealing with biodistribution of 68Ga-PSMA-11 (7,10,25). Taken together, our findings can be interpreted as sufficient evidence that administration of furosemide shortly after tracer injection does not have a relevant negative influence on organ distribution of 68Ga-PSMA-11.
A negative impact of early furosemide injection on lesion detectability could not be observed either. The patient-based overall detection rate of at least 1 68Ga-PSMA-11–avid lesion consistent with recurrent PC was even higher in the group receiving furosemide than in patients without preparation (66.8% vs. 60.5% of cases). In comparison with other studies, a somewhat lower overall detection rate in our analysis may be noticed. In a study by Afshar-Oromieh et al. including 1,007 PC patients with BR referred for 68Ga-PSMA-11 PET/CT, a PET positivity in 79.5% of patients is described (1). In a recently published study by Chevalme et al. on the performance of 68Ga-PSMA-11 PET/CT after a negative or equivocal 18F-fluorocholine PET/CT (including 1,084 PC patients with BR), an overall PET positivity rate of 68% of patients was found (26), revealing no significant difference between subgroups of patients with furosemide administered at the time of tracer injection and patients without furosemide. The lower percentage of PET-positive patients in our analysis may be due to relatively low PSA values of patients in both groups (median PSA: 1.3 ng/mL and 0.82 ng/mL, respectively). It is well known that detection efficacy of 68Ga-PSMA-11 PET/CT increases with higher PSA levels (1,4).
Although with the present study we could demonstrate that furosemide administered at the time of tracer injection has a major impact on assessment of LR in PC patients with BR, a debate on the best time point for injection of furosemide in 68Ga-PSMA-11 PET/CT may remain. Applying a biphasic scan protocol, Afshar-Oromieh et al. described that a reduction of radioactivity in the urinary bladder between scans 1 h after injection and 3 h after injection was more intense after injection of furosemide, assuming a better visibility of PC lesions in the vicinity of the urinary bladder (17). Another approach with late furosemide injection is described by Haupt et al. (16). Compared with a standard protocol without furosemide and scan acquisition 60 min after injection, a slightly higher detection rate of LR was demonstrated in patients orally hydrated with 1 L of water 30 min after injection and injected with furosemide 60 min after injection followed by a single PET examination 90 min after injection (LR present in 42.9% of patients with furosemide vs. 42.0% of patients without furosemide). Haupt et al. demonstrated that urinary bladder activity was significantly lower with furosemide than with the standard protocol, resulting also in a significantly better contrast between LR and urinary bladder activity. The high percentage of LR in both groups in this study is striking and may be explained by the relatively high overall PSA levels of patients analyzed (39.2% of patients with PSA > 4.0 ng/mL). In a study by Derlin et al. using 68Ga-PSMA I&T, a different PSMA-ligand with high urinary bladder accumulation, the authors showed that furosemide injected simultaneously with radiotracer significantly reduced urinary bladder activity on PET scans at 60 min after injection compared with patients without furosemide, resulting also in a significant improvement in assessment of the prostatic fossa (18). However, with respect to evaluation of the prostate bed, best results were obtained with delayed imaging 180 min after injection, performed after oral hydration and injection of furosemide after a PET/CT scan at 60 min after injection.
Despite the favorable results of these furosemide protocols, the described procedures require either a longer waiting time for the patients or additional scans. For institutions with high throughput of PET examinations and limited camera availability a protocol with an uptake time of only 60 min and a single image acquisition as presented in this study is clearly advantageous.
There are some limitations within this study. First, the data were collected retrospectively. We are aware that some parameters in the patient population differed significantly between both preparation groups, such as median radiotracer uptake time, number of patients with primary radiotherapy, and initial T-stage. We cannot exclude that this heterogeneity may have influenced the results. With respect to median uptake time, a difference of 2 min does not, in our view, have a relevant negative effect on results. In particular, Q1 and Q3 demonstrate that most participants had an uptake time between quite a small time window of 60 and 77 min. A major drawback of the current study is that no histologic verification of lesions rated as LR was performed. Usually, biopsy of PSMA-positive LR is not part of our standard clinical work-up of PC patients with BR. However, interpretation of findings was done by 2 experienced readers in a standardized way following published guidelines (8,22). Therefore, we are strongly convinced that PSMA-positive local findings in the prostate fossa, that were not verified by other imaging modalities or on follow-up, can be regarded as true-positive.
CONCLUSION
In PC patients with BR referred for 68Ga-PSMA-11 PET/CT, application of a forced diuresis protocol with 500 mL of NaCl 0.9% and 20 mg of furosemide, injected simultaneously with the radiotracer, has the potential to significantly increase the detection rate of LRs. Moreover, early injection of furosemide does not seem to have a negative influence on organ distribution of 68Ga-PSMA-11 and does not impair lesion detectability of the examination.
DISCLOSURE
No potential conflict of interest relevant to this article was reported.
KEY POINTS
QUESTION: Does intravenous administration of furosemide increase detection rate of LR in PC patients with BR referred for 68Ga-PSMA-11 PET/CT?
PERTINENT FINDINGS: In a retrospective analysis of 440 PC patients undergoing 68Ga-PSMA-11 PET/CT for BR, detection rate of LR is significantly higher in patients injected with 20 mg of furosemide at the time point of tracer administration in comparison with patients receiving no furosemide.
IMPLICATIONS FOR PATIENT CARE: A more accurate assessment of the prostatic fossa after definitive primary therapy has a major impact on therapeutic management in PC patients with BR, especially in those patients in whom salvage radiation therapy is considered.
REFERENCES
- 1. Afshar-Oromieh A, Holland-Letz T, Giesel FL, et al. Diagnostic performance of 68Ga-PSMA-11 (HBED-CC) PET/CT in patients with recurrent prostate cancer: evaluation in 1007 patients. Eur J Nucl Med Mol Imaging. 2017;44:1258–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Caroli P, Sandler I, Matteucci F, et al. 68Ga-PSMA PET/CT in patients with recurrent prostate cancer after radical treatment: prospective results in 314 patients. Eur J Nucl Med Mol Imaging. 2018;45:2035–2044. [DOI] [PubMed] [Google Scholar]
- 3. Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol. 2019;5:856–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eiber M, Maurer T, Souvatzoglou M, et al. Evaluation of hybrid 68Ga-PSMA ligand PET/CT in 248 patients with biochemical recurrence after radical prostatectomy. J Nucl Med. 2015;56:668–674. [DOI] [PubMed] [Google Scholar]
- 5. Ceci F, Uprimny C, Nilica B, et al. 68Ga-PSMA PET/CT for restaging recurrent prostate cancer: which factors are associated with PET/CT detection rate? Eur J Nucl Med Mol Imaging. 2015;42:1284–1294. [DOI] [PubMed] [Google Scholar]
- 6. Fendler WP, Eiber M, Beheshti M, et al. 68Ga-PSMA PET/CT: joint EANM and SNMMI procedure guideline for prostate cancer imaging: version 1.0. Eur J Nucl Med Mol Imaging. 2017;44:1014–1024. [DOI] [PubMed] [Google Scholar]
- 7. Afshar-Oromieh A, Malcher A, Eder M, et al. PET imaging with a (68Ga)Gallium-labelled PSMA ligand for the diagnosis of prostate cancer: biodistribution in humans and first evaluation of tumour lesions. Eur J Nucl Med Mol Imaging. 2013;40:486–495. [DOI] [PubMed] [Google Scholar]
- 8. Fanti S, Minozzi S, Morigi JJ, et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur J Nucl Med Mol Imaging. 2017;44:1622–1635. [DOI] [PubMed] [Google Scholar]
- 9. Afshar-Oromieh A, Haberkorn U, Schlemmer HP, et al. Comparison of PET/CT and PET/MRI hybrid systems using a 68Ga-labelled PSMA ligand for the diagnosis of recurrent prostate cancer: initial experience. Eur J Nucl Med Mol Imaging. 2014;41:887–897. [DOI] [PubMed] [Google Scholar]
- 10. Hofman MS, Hicks RJ, Maurer T, Eiber M. Prostate-specific membrane antigen PET: clinical utility in prostate cancer, normal patterns, pearls, and pitfalls. Radiographics. 2018;38:200–217. [DOI] [PubMed] [Google Scholar]
- 11. Freitag MT, Radtke JP, Afshar-Oromieh A, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging. 2017;44:776–787. [DOI] [PubMed] [Google Scholar]
- 12. Rahbar K, Weckesser M, Ahmadzadehfar H, Schäfers M, Stegger L, Bögemann M. Advantage of 18F-PSMA-1007 over 68Ga-PSMA-11 PET imaging for differentiation of local recurrence vs. urinary tracer excretion. Eur J Nucl Med Mol Imaging. 2018;45:1076–1077. [DOI] [PubMed] [Google Scholar]
- 13. Uprimny C, Kroiss AS, Fritz J, et al. Early PET imaging with 68Ga-PSMA-11 increases the detection rate of local recurrence in prostate cancer patients with biochemical recurrence. Eur J Nucl Med Mol Imaging. 2017;44:1647–1655. [DOI] [PubMed] [Google Scholar]
- 14. Uprimny C, Kroiss AS, Decristoforo C, et al. Early dynamic imaging in 68Ga-PSMA-11 PET/CT allows discrimination of urinary bladder activity and prostate cancer lesions. Eur J Nucl Med Mol Imaging. 2017;44:765–775. [DOI] [PubMed] [Google Scholar]
- 15. Fennessy N, Lee J, Shin J, et al. Frusemide aids diagnostic interpretation of (68Ga)-PSMA positron emission tomography/CT in men with prostate cancer. J Med Imaging Radiat Oncol. 2017;61:739–744. [DOI] [PubMed] [Google Scholar]
- 16. Haupt F, Dijkstra L, Alberts I, et al. 68Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer - a modified protocol compared with the common protocol. Eur J Nucl Med Mol Imaging. 2020;47:624–631. [DOI] [PubMed] [Google Scholar]
- 17. Afshar-Oromieh A, Sattler LP, Mier W, et al. The clinical impact of additional late PET/CT imaging with 68Ga-PSMA-11 (HBED-CC) in the diagnosis of prostate cancer. J Nucl Med. 2017;58:750–755. [DOI] [PubMed] [Google Scholar]
- 18. Derlin T, Weiberg D, von Klot C, et al. 68Ga-PSMA I&T PET/CT for assessment of prostate cancer: evaluation of image quality after forced diuresis and delayed imaging. Eur Radiol. 2016;26:4345–4353. [DOI] [PubMed] [Google Scholar]
- 19. Uprimny C, Bayerschmidt S, Kroiss AS, et al. Impact of forced diuresis with furosemide and hydration on the halo artefact and intensity of tracer accumulation in the urinary bladder and kidneys on (68Ga)Ga-PSMA-11-PET/CT in the evaluation of prostate cancer patients. Eur J Nucl Med Mol Imaging. 2021;48:123–133. [DOI] [PubMed] [Google Scholar]
- 20. World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. [DOI] [PubMed] [Google Scholar]
- 21. Uprimny C, Kroiss AS, Decristoforo C, et al. 68Ga-PSMA-11 PET/CT in primary staging of prostate cancer: PSA and Gleason score predict the intensity of tracer accumulation in the primary tumour. Eur J Nucl Med Mol Imaging. 2017;44:941–949. [DOI] [PubMed] [Google Scholar]
- 22. Eiber M, Herrmann K, Calais J, et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): proposed miTNM classification for the interpretation of PSMA-ligand PET/CT. J Nucl Med. 2018;59:469–478. [DOI] [PubMed] [Google Scholar]
- 23.R: A language and environment for statistical computing. Vienna, Austria: R Foundation of Statistical Computing; 2018. [Google Scholar]
- 24. Perveen G, Arora G, Damle NA, et al. Can early dynamic positron emission tomography/computed tomography obviate the need for postdiuresis image in 68Ga-PSMA-HBED-CC scan for evaluation of prostate adenocarcinoma? Indian J Nucl Med. 2018;33:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Prasad V, Steffen IG, Diederichs G, Makowski MR, Wust P, Brenner W. Biodistribution of (68Ga)PSMA-HBED-CC in patients with prostate cancer: characterization of uptake in normal organs and tumour lesions. Mol Imaging Biol. 2016;18:428–436. [DOI] [PubMed] [Google Scholar]
- 26. Chevalme Y-M, Boudali L, Gauthé M, et al. Survey by the French Medicine Agency (ANSM) of the imaging protocol, detection rate, and safety of 68Ga-PSMA-11 PET/CT in the biochemical recurrence of prostate cancer in case of negative or equivocal 18F-fluorocholine PET/CT: 1084 examinations. Eur J Nucl Med Mol Imaging. 2021;48:2935–2950. [DOI] [PMC free article] [PubMed] [Google Scholar]