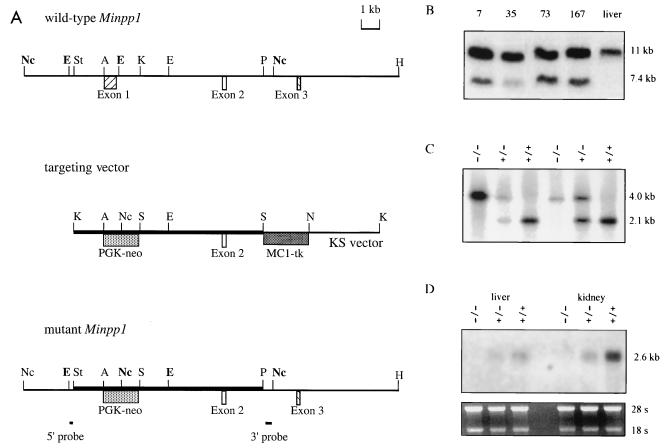
FIG. 2.
Targeted deletion of Minpp1 by homologous recombination. (A) Targeting strategy. Homologous recombination between the Minpp1 wild-type allele (top) and the targeting vector (middle) substitutes neo for a 2-kb endogenous sequence containing all of the coding sequence of exon 1 and part of intron 1 (bottom). Two probes for screening ES cells and genotyping mice are also shown: 5′ probe, a 0.18-kb EcoRI-StyI fragment; 3′ probe, a 0.4-kb PstI-NcoI fragment. Boldface EcoRI and NcoI sites mark the sizes of the hybridized fragments on Southern blots. Nc, NcoI; E, EcoRI; St, StyI; A, ApaI; K, KpnI; P, PstI; H, HindIII; S, SalI; N, NotI. (B) Southern blot analysis of ES cell clones. DNA was digested with NcoI and hybridized to the 3′ probe. The wild-type allele is 11.0 kb in length, and the mutant allele is 7.4 kb. Shown are four targeted ES cell clones and control liver DNA from a wild-type mouse. (C) Southern blot analysis of EcoRI-digested mouse tail DNA. The 5′ probe detected a 2.1-kb fragment from the wild-type allele and a 4.0-kb fragment from the mutant allele. Mice of all genotypes were identified from the Minpp1 heterozygous crosses. +/+, wild type; +/−, heterozygous mutant; −/−, homozygous mutant. (D) Northern blot analysis of liver and kidney RNA from Minpp1+/+, Minpp1+/−, and Minpp1−/− mice with a Minpp1 cDNA probe containing all of the protein coding sequence. Minpp1 message was undetectable in the Minpp1−/− tissues (top). rRNAs stained with ethidium bromide were used as loading controls (bottom).