Abstract
Recent evidence suggests that altered responses to affective touch—a pleasant interoceptive stimulus associated with activation of the C-Tactile (CT) system—may contribute to the aetiology and maintenance of mental conditions characterised by body image disturbances (e.g., Anorexia Nervosa). Here, we investigated whether tactile pleasantness and intensity differ across body sites, and if individual differences in dysmorphic appearance concerns and body and emotional awareness might be associated with touch perceptions across body sites. To this end, we measured perceived pleasantness and intensity of gentle, dynamic stroking touches applied to the palm, forearm, face, abdomen and back of 30 female participants (mean age: 25.87±1.17yrs) using CT-optimal (3 cm/s) and non-CT optimal (0.3 and 30 cm/s) stroking touch. As expected, participants rated CT-targeted touch as more pleasant compared to the two non-CT optimal stroking touch at all body sites. Regardless of stroking velocity, touch applied to the abdomen elicited the lowest pleasantness ratings. Lower levels of emotional awareness, greater levels of interoceptive sensibility and of dysmorphic concerns were associated with lower preference for CT-optimal stroking touch applied to the forearm and the back. These findings begin to elucidate the link between CT sensitivity, dysmorphic appearance concerns and body and emotional awareness, which may have implications for future research looking to inform early interventions. Addressing impaired processing of affective interoceptive stimuli, such as CT-targeted touch, may be the key to current treatment approaches available for those populations at risk of disorders characterised by body image disturbance.
Introduction
Touch is a crucial means of receiving information from the outside world by mediating our interactions with objects, and other individuals. The touch experience is a combination of the discriminative aspects of tactile perception (i.e. characterising and localising external stimuli), and the affective and social qualities encoded therein [1, 2]. Previous investigations have demonstrated that slow, caress-like touch is usually experienced as highly pleasant [3–6] and plays a pivotal role in instigating and maintaining close social interactions.
It is hypothesised that social and affective qualities of touch are mediated by a specific class of unmyelinated mechanoreceptors–C-tactile afferents (CTs) [7–9]—typically found in hairy, but not glabrous skin. Neurophysiological investigations by means of single-unit microneurography have shown that CTs are velocity tuned, responding optimally to low force stroking velocities (~ 3cm/s), delivered at skin temperature [10]. The relationship between CT activation and stroke velocity is best described by an inverted U-shaped regression, with the greatest response at 3 cm/s, and weaker responses at slower (0.1 cm/s) and faster velocities (30 cm/s) [3, 4]. Importantly, this response pattern strongly correlates with subjective ratings of stimulus pleasantness and velocity preference [3].
According to the ‘Social Touch hypothesis’ [1] CTs, which carry information about the ‘hedonic’ aspect of interpersonal, gentle touch, have evolved to promote social bonding. A multitude of research has corroborated their importance in affiliative interactions. It has been shown that, when asked to stroke their partners or babies, people spontaneously delivered touch at CT-optimal velocities [11]. Moreover, CTs optimally respond to touch delivered at skin temperature [5]. Consistent with the social touch hypothesis, CT-optimal touch was also found to have physiological, soothing effects [12–14], and health benefits, including stress relief associated with a decrease in heart rate, blood pressure, and cortisol release [15]. For example, recent psychophysiological investigations have shown that CT-optimal stroking to the forearm of 9-month old infants selectively decreased heart rate [14] and that CT-targeted touch produces a significant decrease in preterm infants’ heart-rates and increase in their blood oxygenation levels [15]. Furthermore, a recent study [16] reported that affective touch reduced the distress caused by social exclusion.
The primary cortical target for CT projections is the posterior Insula [17, 18]. Accordingly, fMRI studies have shown that affective touch elicits a velocity-specific hemodynamic response in the contralateral posterior Insula [17–21], and in higher-order regions such as orbitofrontal cortices [22, 23]. The Insula is also thought to sustain the early convergence of sensory and affective signals about the body, contributing to so-called ‘interoceptive processing’ [18, 24]. According to Craig [24], interoception refers to the awareness of the physiological condition of the body, which integrates both sensations coming from within the body, (e.g. cardiac, respiratory, and digestive signals), and from the outside (e.g., temperature, itch, pain, and pleasure). In turn, the processing and integration of interoceptive information contributes to the maintenance of body awareness and to the regulation of homeostasis [25, 26]. CT projections to insular brain regions characterize these afferents as an interoceptive modality, therefore conveying information about the affective and physiological state of the body, and ultimately contributing to body awareness [18, 27]. Top-down influences (e.g., the learnt affective and social meanings of interpersonal touch) can affect the hedonic value of touch leading to a high inter-individual variability in tactile pleasantness [28]. For example, the motivational style and gender of the touch receiver might influence the perceived pleasantness of interpersonal touch [29]. Differences based on individual’s social abilities, as measured by autistic traits [30] or attachment style [31], may also alter social touch perception. Moreover, recent research has shown that touch elicits lower subjective pleasantness ratings in individuals with Anorexia Nervosa (AN), a trait that endures even after an otherwise successful recovery [32, 33]. Anorexia Nervosa is a psychiatric condition characterised by profound disturbances in eating behaviours and body image, that is an individual’s conscious perception of, and attitude towards, their bodily appearance [34, 35], accompanied by impairments in social cognition and by altered perception of interoceptive bodily sensations [36–38]. These alterations might also extend to the interoceptive modality of affective touch, indicating the presence of a bodily-encoded anhedonia in interpersonal interactions [33].
Further studies have recently shown that both acute and recovered AN (RAN) patients also display a reduced responsivity to the anticipation of CT-optimal touch, both in terms of neural response localized to the ventral mid-insula, and in terms of predicted pleasantness of touch [33, 39]. Taken together, these findings suggest that in AN there is a dysregulation in the ability to correctly predict and interpret interoceptive stimuli, including CT-based pleasantness of touch.
Different lines of research have shown that body perception in Eating Disorders (EDs) is characterised by an over-investment on external physical features of the body (i.e., body image concerns) coupled with altered perception of information arising from within the body (i.e., interoception) [40, 41]. Accordingly, research in clinical and non-clinical populations has shown the existence of a link between body dissatisfaction, physical appearance pre-occupation and interoceptive deficits [38, 42–45]. Yet, this would be the case for all psychiatric conditions in which the body is a motive for concerns, including not only EDs but also, for example, Body Dysmorphic Disorder (BDD) [46, 47]. Like EDs, individuals with BDD typically exhibit a distorted image of their bodies and preoccupation with their appearance and shape [48]. Despite the fact that somatic misperceptions and low levels of interoceptive awareness have been included as part of a theoretical model of BDD [49], there are no studies which attest the putative role of CTs in such clinical conditions, a question which warrants further investigation.
The purpose of this study was threefold. First, we examined whether pleasantness of touch varies across different hairy skin body sites that are innervated by CTs. In other mammals these nerves are called C-low threshold mechanoreceptors (CLTM) and in a study with mice it was shown that CLTMs more densely innervate proximal body sites, such as the back and head, compared to distal sites [50]. In a recent study with humans where participants viewed video clips of touch to different body sites and were asked how pleasant they thought the touch was to the receiver, sites that are more proximal were also reported as being more pleasant [51]. However, the classical approach for characterising CT (actual) responses has usually limited its focus to the forearm, compared to the glabrous skin of the palm, which should be CT-free. To the best of our knowledge, only three studies have looked so far at differences in pleasantness ratings at several stroking velocities, across several skin sites [3, 4, 52], thus characterising the pleasantness/stroking velocity profile for each of these body sites. In the current study, we wanted to replicate and extend previous findings by targeting additional body sites to include the cheek, back and abdomen.
Second, we wanted to explore whether EDs and BDD traits modulate the way touch is experienced in the above-mentioned body sites. Previous psychological studies targeting AN population have mostly focussed on the somatosensory (discriminative) aspect of tactile perception only (mainly processed by large diameter, fast conducting, myelinated Aβ afferents) [53, 54]. For example, Keizer et al. [55–57] used a 2-point discrimination (2pd) task and reported that AN patients overestimate 2pd distances when presented to both sensitive and insensitive body sites, such as the abdomen and the forearm, respectively. Furthermore, another study by Spitoni et al. [58], which measured 2pd thresholds, found that AN patients showed relative overestimation of tactile distances on the abdomen compared to the sternum. Whilst overall these findings seem to provide evidence of an impairment of the somatosensory, discriminatory aspect of touch at emotionally salient body sites and its link to body image disturbance, no studies so far investigated whether responses to affective touch in the presence of EDs and BDD traits are modulated by specific body sites with a possible negative emotional valence. To this aim, we targeted for the first time, an emotionally salient body site with a negative valence, i.e., the abdomen, which is usually linked to worry of fatness and dissatisfaction in the general population [59, 60] and in individuals experiencing EDs [61–65]. Furthermore, we targeted the face area as it represents a body site which is salient for the construction of one’s own identity and body image [66], and also because individuals suffering from BDD commonly experience concerns about their facial appearance [67–69].
Third, given the importance of interoceptive awareness in one’s sense of self, we were also interested in exploring the relationships between subjective experience of pleasant touch at the different body sites with self-reports of interoceptive sensibility (as measured by the Body Perception Questionnaire, BPQ) [70] and of emotional awareness (as measured using the interoceptive deficits scale of the Eating Disorder Inventory-3, EDI-3) [71]—two separate interoceptive components associated with EDs and BDD [46, 47, 72, 73]. To do so, we employed an affective touch manipulation task during which participants were required to provide pleasantness ratings of light, dynamic stroking touches applied to five body sites: palm, face, abdomen, back and forearm. One velocity was targeted to optimally activate CTs (3 cm/s) whereas the other two, faster (30 cm/s) and slower (0.3 cm/s) strokes fell outside the CT optimal range [3].
Furthermore, we collected ratings about participants’ touch intensity experience at the varying body sites. With these regards, recent evidence suggests subjective evaluations of touch responses, such as intensity ratings, are not velocity specific in the same way as pleasantness ratings [74, 75], and that recovered anorexics display lower insular cortex responses during touch anticipation associated with higher perceived touch intensity ratings [39]. Therefore, we collected participants’ ratings of touch intensity, to account for potential individual differences in the two aspects of touch (discriminative vs. affective).
Overall, we expected that differences in touch velocities and application sites would inform the degree to which responses to predominantly discriminatory (touch to the palm and non-CT optimal touch) compared to affective/CT-activating (e.g., CT targeted 3 cm/s touch to the cheek, abdomen, back and forearm) tactile stimuli are associated with dysmorphic appearance concerns and body and emotional awareness. Based on previous research investigating the relationship between touch pleasantness and stroking velocity at the forearm [3], we predicted that participants would prefer the CT-optimal (3cm/s) stroking velocity, with reduced pleasantness for non-CT optimal (0.3 cm/s) slower stroking velocity and the non-CT optimal (30 cm/s) faster stroking velocity. Crucially, based on previous findings from clinical populations [32, 76 but see 77 and 78, for opposite results in two samples of healthy women], we also expected to observe a significant relationship between dysmorphic appearance concerns and pleasant touch awareness, particularly at emotionally salient body sites (for e.g., abdomen and face, as compared to the palm). Finally, we hypothesised that self-reports of body and emotional awareness would be related to pleasant touch awareness perception, so that individuals’ (hyper)sensitivity to internal bodily sensations and higher emotional awareness will be predictive of participants’ preference of CT-optimal (3cm/s) touch. At the same time, the sensory, touch intensity, aspect of touch was expected to differ across the different body sites and to be associated with individual differences in dysmorphic appearance concerns and body awareness.
Methods
Participants
The sample size was based on a preliminary calculation using the freely available G*Power software (G*Power 3.1.9.4) [79], which indicated a minimum sample of 16 participants as adequate for a design with 95% power to detect a moderate effect size (f = 0.25), using a repeated-measures ANOVA with alpha at .05 (two tailed). To cope with potential drop out and outlier case exclusion, a total of 30 women (mean age = 25.87±1.17yrs; mean BMI = 27.22±1.06kg/m2) were recruited. With regards to the (exploratory) multiple regression analyses (see Data Handling section), a post-hoc power analysis using G*Power 3.1.9.4 indicated that (given the total sample size = 30; number of predictors = 4; squared multiple correlation p2 = 0.376) the power to detect obtained effects at the alpha level of .05 was .89, for the overall regression in prediction of the forearm’ PTA. Similarly, the post-hoc G*Power analysis relative to the overall regression in prediction of the back’ PTA indicated that (given the total sample size = 30; number of predictors = 4; squared multiple correlation p2 = 0.321) the power to detect obtained effects at the alpha level of .05 was .79.
Participants were recruited internally through the Liverpool John Moores University (LJMU) research participation system for undergraduate Psychology students in exchange for course credits and externally through poster advertisements situated in public locations, social media and through individuals known to the researchers. In line with previous literature analysing body perception and eating behaviours, the current study employed a population of only female participants [60, 80, 81]. Indeed, literature on the prevalence and the phenomenology of EDs in male populations is still limited [82]. All participants, except one, were right-handed as assessed by the Edinburgh Handedness Inventory [83]. All reported normal or corrected to normal vision and they were in good health, free of psychotropic or vasoactive medication, with no current or history of any psychiatric or neurological disease, no skin conditions (e.g. psoriasis, eczema, etc.) and not pregnant. Participants provided written informed consent prior to testing and were debriefed at the end of the experiment. All procedures were approved by the Research Ethics University Committee (UREC, approval n.: 19NSP009) of Liverpool John Moores University and complied with the ethical standards of the 1964 Declaration of Helsinki.
Self-report questionnaires
Eating Disorder Inventory-3
The Eating Disorder Inventory-3 (EDI‐3) [71] comprises 91 items assessing ED core symptoms (i.e., Drive for Thinness, Bulimia and Body Dissatisfaction), as well as other psychological constructs and personality traits that have been associated with disordered eating behaviours (Low Self-esteem, Personal Alienation, Interpersonal Insecurity, Interpersonal Alienation, Interoceptive Deficits, Emotional Dysregulation, Perfectionism, Ascetism and Maturity Fear). Examples of items of this scale include items such as “I think my hips are too big”, “I am preoccupied with the desire to be thinner” “I feel alone in the world” and “I stuff myself with food”. Participants are instructed to rate each item on a 6‐point Likert scale ranging from ‘never’ to ‘always’. The EDI-3 allows the calculation of 12 scales and 6 composites describing different aspects of the ED symptomatology.
In the current study, we were specifically interested in assessing whether participants’ overall level of ED symptoms and of emotional awareness could influence their experience of pleasantness and intensity related to touch. To this aim, we focussed our analyses on the ED Risk Composite (EDRC) and the Interoceptive Deficits subscale of the EDI-3. The EDRC score represents an index of the risk to develop an ED and is calculated by summing the scores of the three symptom-specific subscales of the inventory: Drive for Thinness, Bulimia and Body Dissatisfaction. The Interoceptive Deficits subscale assesses ‘confusion related to accurately recognizing and responding to emotional states’ [71] and it is considered a measure of emotional (rather than somatic) awareness [42, 73]. The EDI-3 was validated in clinical and non‐clinical samples and has shown good internal consistency (α = between .75 and .92 for each subscale), and excellent sensitivity and specificity [84]. In this particular sample, the EDI-3 scale had excellent internal consistency (Cronbach’s alpha = .94).
Dysmorphic Concern Questionnaire
The Dysmorphic Concern Questionnaire (DCQ) [85] comprises 7 items investigating participants’ concern about their physical appearance. The scale has been validated as a brief, sensitive, and specific screening measure of trait-level BDD symptoms [86]. Items focus on the belief of being misshapen or malformed despite others’ opinion; belief in bodily malfunction (e.g. malodour); consultation with cosmetic specialists; spending excessive time worrying about appearance; and spending a lot of time covering up perceived defects in appearance. Participants were asked to rate each item on a Likert scale from a minimum of 0 (“not at all”) to a maximum of 4 (“much more than most people”). Total scores range from 0 to 28 with a critical value of 9 indicating clinical concern [86]. The scale was administered to investigate whether individual differences in physical appearance concerns (higher trait BDD symptoms) could explain participants’ responses to CT-optimal touch at the different body sites. The DCQ has been shown good internal consistency with α = .80 [87]. In this particular sample, the DCQ scale had good internal consistency (Cronbach’s alpha = .88).
Body Perception Questionnaire-Short Form. The Body Perception Questionnaire-Short Form (BPQ-SF) [70] is a 46-item self-report questionnaire for the assessment of participants’ awareness about different bodily states associated with changes in the activity of the autonomic nervous system, such as “muscle tension”, “goose bumps”, “stomach and gut pains”, breathing and heart-beat rates. Items are rated on a 5-point Likert scale ranging from 1 (“Never”) to 5 (“Always”), and total scores ranging between 12 and 60. In the current study, we focussed on the Body Awareness subscale of the BPQ (26 items) which reflects participants’ sensitivity to inner bodily sensations, with higher values reflecting a hypersensitivity and lower values a hyposensitivity to bodily sensations. Together with the Interoceptive Deficit subscale of the EDI-3, the BPQ was administered to investigate whether individual differences in body awareness could explain differences in participants’ responses to CT-optimal touch at different body sites. The BPQ has been validated in different samples and has been shown to have good internal consistency (α = between .88 and .97), and an excellent test-retest reliability [88]. In this particular sample, the BPQ scale had excellent internal consistency (Cronbach’s alpha = .96).
Body Mass Index
Each participant’s body mass index (BMI) was physically calculated on the day of the experimental session, from their weight and height obtained from a calibrated digital scale (OMRON BF511) and a stadiometer.
General procedure. Participants were lying semi-horizontally in a comfortable, reclining dental chair whilst receiving manual brush strokes. They were instructed to remain still with their arms resting on the chair armrest. Although during each trial participants kept their eyes open, the position on the chair prevented participants from seeing the stroking procedure at the varying body sites.
During the experiment, participants received manual brush strokes to the dorsal surface of their (exposed) forearm, upper part of the back, stomach, cheek and palm of their right body (in a randomized order) using a soft cosmetic brush (No7 cosmetic brush, Boots UK). Each stroking area measured 9 cm long and was identified and marked with a washable marker on participants’ skin, so to provide a specific area for which to administer tactile stimulation for participants. To avoid any social confound, stroking touch was delivered by the same female experimenter to all participants (SSh). During all experimental trials, the experimenter stood by the participants, to the right of the reclining dental chair.
During each trial, strokes were delivered for 6 secs on a 9 cm surface at one of three set velocities: 0.3 cm/s, 3 cm/s, and 30 cm/s. The experimenter was trained to deliver stroking touch with a constant pressure of 220 mN, which was calibrated using a high precision digital scale. The stroking was delivered in a proximal to distal direction.
Overall, the stroking task consisted of 5 blocks: one block for each body site. Each block consisted of 9 trials, with 3 trials for each velocity. Across the 5 blocks, participants experienced a total of 15 CT-optimal trials (3 cm/s, 2 strokes per trial), 15 non-CT optimal fast touch trials (30 cm/s, 20 strokes per trial) and 15 non-CT optimal slow touch trials (0.3 cm/s, 1/5 of a stroke per trial corresponding to 1.8 cm). Participants were randomly assigned to receive the experimental blocks in one of 5 pseudo-randomised orders, which ensured no two consecutive trials were the same.
The running orders were created in PsychoPy [89]. Trial velocity was randomised to minimize habituation [90]. Trials were delivered 30s apart, as CT fibers are easily fatigued [9].
A visual metronome, programmed in PsychoPy, was presented on a computer screen behind the participant [91, 92] and guided the researcher in delivering the brush strokes at one of the three velocities.
After each trial, by using their left hand, participants rated, on a paper, the pleasantness and intensity of the stimulation on 15 cm horizontal Visual Analogue Scales (VAS) ranging respectively from “unpleasant” to “pleasant”, and from “least intense” to “most intense” (see Fig 1).
Fig 1. Schematic depiction of the study procedure.
Participants were lying semi-horizontally in a comfortable, reclining chair whilst receiving manual brush strokes. In randomised blocks (each one corresponding to one body site), participants were asked to rate the pleasantness and intensity of the touch delivered by a female experimenter to the palm, face, abdomen, back and forearm at 3 cm/s (CT-optimal stroking) and 0.3 cm/s and 30 cm/s (non-CT optimal stroking) velocities.
At the end of the experiment, participants were weighted on the digital scale and were moved to a desk where they were asked to complete the self-report questionnaires.
Data handling
All statistical analyses were performed using STATISTICA 8.0 (StatSoftInc, Tulsa, Oklahoma). In our main analysis, mean pleasantness and intensity ratings were entered into two separate two-way 5×3 repeated-measures Analysis of Variance (ANOVA) with body sites (palm, face, abdomen, back and forearm) and stroking velocity (0.3 cm/s, 3 cm/s, 30 cm/s) as within-subjects factors. All data are reported as Mean (M) and Standard Error of the Mean (S.E.M.). A significance threshold of p < 0.05 was set for all effects and effect sizes were estimated using the partial eta square measure (ηp2). Duncan post-hoc tests were performed to follow-up significant interactions. Furthermore, following previous studies [6, 93], we computed two main affective touch indices, as follows: 1) overall touch intensity (OTI) which is calculated as the mean of all touch intensity ratings and 2) pleasant touch awareness (PTA) which is calculated as the difference of pleasantness ratings between CT-optimal (3 cm/s) and non-CT optimal (30 cm/s) fast stroking, weighted by the mean of all touch pleasantness ratings (overall touch pleasantness), [PTA = (pleasantness ratings at 3 cm/s−pleasantness ratings at 30 cm/s)/overall touch pleasantness]. A positive PTA index indicates that participants prefer stroking at CT-optimal 3 cm/s velocity to stroking at non-CT optimal (30 cm/s) fast velocity. To explore the associations of these two indices with individual scores obtained at the self-report scales: EDRC (EDI-3), DCQ, Interoceptive Deficits (EDI-3) and Body awareness (BPQ), a series of multiple linear regressions were fit to predict the two measures of touch experience at each of the five body sites.
Results
Demographics and self-report scales
Participants’ demographics and self-report questionnaire scores are reported in Table 1. The mean BMI for our sample was 27.22, which is also comparable to the most recent United Kingdom averages for women (M = 27.2; National Health Service Digital, 2016). The mean EDRC score was 18.53 (±1.41, range: 1–29) which is deemed to be at low risk for the development of an ED, with scores above 30 indicating clinical levels of disordered eating [84]. The mean DCQ score was 7.67 (±0.80, range: 1–16) which is below the critical level of high dysmorphic concerns, with scores above 9 indicating clinical levels of body image concerns [85].
Table 1. Participants’ means and standard error of means (S.E.M. in brackets) of demographic variables and self-report questionnaire scores.
| Mean (s.e.m.) | Minimum | Maximum | |
|---|---|---|---|
| Age (yrs) | 25.87 (1.17) | 21 | 40 |
| BMI (kg/cm2) | 27.22 (1.06) | 18 | 40.7 |
| EDI-3 | |||
| Drive for Thinness | 8.13 (0.78) | 0 | 16 |
| Body Dissatisfaction | 7.33 (0.64) | 0 | 12 |
| Bulimia | 3.07 (0.45) | 0 | 8 |
| Low self esteem | 1 (0.21) | 0 | 4 |
| Personal Alienation | 5.83 (0.76) | 0 | 15 |
| Interpersonal Insecurity | 7.3 (0.92) | 0 | 17 |
| Interpersonal Alienation | 6.7 (0.91) | 0 | 20 |
| Interoceptive Deficit | 7.57 (1.10) | 0 | 24 |
| Emotional Dysregulation | 8.03 (1.14) | 0 | 24 |
| Perfectionism | 10.03 (1.12) | 0 | 23 |
| Ascetism | 7 (1.01) | 0 | 21 |
| Maturity Fear | 9.67 (1.16) | 1 | 28 |
| EDCR | 18.53 (1.41) | 1 | 29 |
| DCQ | 7.67 (0.80) | 1 | 16 |
| Body Awareness (BPQ) | 63.17 (4.13) | 29 | 112 |
Notes: BMI, Body Mass Index; EDI-3, Eating Disorder Inventory-3; EDCR, Eating Disorder Risk Composite; DCQ, Dysmorphic Concern Questionnaire; BPQ, Body Perception Questionnaire.
Main analysis
Pleasantness ratings
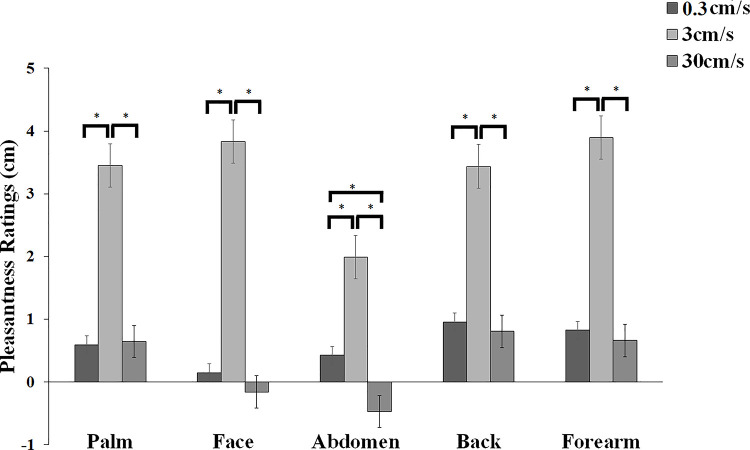
The 2-way ANOVA of pleasantness ratings revealed a significant main effect of body sites [F(4,116) = 4.475, p = 0.002, ηp2 = 0.134] with touch to the abdomen rated significantly lower (all ps < 0.047) than any other body site. No significant differences were observed amongst all other body sites (all ps> 0.311). There was also a significant main effect of stroking velocity [F(2,58) = 18.706, p < 0.001, ηp2 = 0.392]. Whilst the touch applied at CT-optimal (3 cm/s) stroking velocity was rated significantly more pleasant than the touch applied at the other two non-CT optimal velocities (all ps < 0.001), no difference was observed between the two non-CT optimal (0.3 cm/s vs. 30 cm/s) stroking velocities (p = 0.593). Importantly, the 2-way interaction of body sites and stroking velocity was also significant [F(8,232) = 2.594, p = 0.010, ηp2 = 0.082, see Fig 2]. Post-hoc comparisons showed that whilst there was no difference amongst the palm, face, back and forearm body sites for the preferred CT-optimal (3 cm/s) stroking velocity (all ps>0.314), touch delivered at CT-optimal (3cm/s) velocity to the abdomen was rated significantly less pleasant compared to all body sites (all ps < 0.001). Moreover, whilst participants rated the touch delivered at the non-CT optimal (30 cm/s) velocity as similarly less pleasant than the non-CT optimal (0.3 cm/s) touch at all body sites (all ps>0.697), this was not the case for the abdomen. At this body site, the non-CT optimal (30 cm/s) touch was rated as significantly less pleasant than the non-CT optimal (0.3 cm/s) touch (p = 0.029). However, this effect was not specific for the abdomen, since no significant difference in pleasantness was observed between the abdomen and the face, when the stroking was delivered at non-CT optimal (30cm/s) velocity (p = 0.407). Furthermore, no difference for the non-CT optimal (30cm/s) touch was observed when comparing this velocity amongst the palm, back and forearm (all ps>0.111). However, participants rated the non-CT optimal (30cm/s) touch as less significantly pleasant when delivered to the face and the abdomen (all ps<0.045). Finally, no significant differences in pleasantness were observed when comparing non-CT Optimal (0.3 cm/s) touch delivered at all body sites (all ps>0.394).
Fig 2. Mean pleasantness ratings (+/- s.e.m) for touch delivered at each of five body sites (palm, face (cheek), abdomen, back, forearm) at 3 cm/s (CT-optimal stroking) and 0.3 cm/s and 30 cm/s (non-CT optimal stroking) velocities.
Additionally, separate regression analyses for each body site were carried out to examine whether the relationship between perceived pleasantness and stroking velocities was better described by linear or quadratic models. Results consistently showed at every body site, profiles were all best fit by negative quadratic models (all ps <0.001), rather than linear models (all ps > 0.20), giving the characteristic “inverted-U” shaped curves, as found in previous studies investigating pleasantness of different velocity stroking stimuli [3, 45].
Intensity ratings
The 2-way ANOVA of intensity ratings revealed a significant main effect of body sites [F(4,116) = 6.203, p < 0.001, ηp2 = 0.176], with touch to the back rated significantly less intense than any other body site (all ps < 0.044). No statistical difference in intensity ratings was identified between the other body sites (all ps>0.080). Furthermore, and as expected, the main effect of stroking velocity was significant [F(2,58) = 35.705, p < 0.001, ηp2 = 0.552] with non-CT optimal (30 cm/s) touch being rated as significantly more intense than the CT-Optimal (3 cm/s) and non-CT optimal (0.3 cm/s) touch (all ps < 0.001). No statistical difference was observed between the intensity ratings for the non-CT optimal (0.3 cm/s) touch and the CT-optimal (3cm/s) touch (p = 0.874). The 2-way interaction was not significant [F(8,232) = 0.761, p = 0.637, ηp2 = 0.026, see Fig 3].
Fig 3. Mean intensity ratings (+/- s.e.m) for touch delivered at each of five body sites (palm, face (cheek), abdomen, back, forearm) at 3 cm/s (CT-optimal stroking) and 0.3 cm/s and 30 cm/s (non-CT optimal stroking) velocities.
Exploratory analyses
By means of a series of exploratory (due to the relatively small sample size for such analyses) multiple regression analyses, we assessed whether symptoms of eating and dysmorphic concerns, and of different interoceptive components could predict participants’ PTA and overall touch intensity (OTI) at all body sites. To this aim, we ran separate (simultaneous) multiple regressions to explore whether EDRC (EDI-3), DCQ, interoceptive deficit EDI-3 and interoceptive sensitivity (BPQ) were significant predictors of PTA and overall touch intensity, at each body site.
The linear multiple regression analysis calculated to predict PTA for the back from symptoms of eating and dysmorphic concerns, interoceptive deficits and Body Awareness scores was significant [F(4,25) = 2.959, p = 0.039, R2 = 0.321], with Body Awareness emerging as the only significant predictor (see Table 2). Furthermore, we found a significant regression equation for the forearm with DCQ and Interoceptive deficits scores being significant predictors [F(4,25) = 3.770, p = 0.016, R2 = 0.376, see Table 2]. We found non-significant regression equations for the palm [F(4,25) = 0.787, p = 0.544, R2 = 0.112], face [F(4,25) = 1.204, p = 0.334, R2 = 0.161], and abdomen [F(4,25) = 0.407, p = 0.801, R2 = 0.061]. Full results are reported in Table 2.
Table 2. Unstandardized coefficients from the linear simultaneous multiple regression models of eating and dysmorphic concerns and body awareness predictors of the pleasantness touch awareness (PTA) index at each body site (palm, face, abdomen, back and forearm).
| Pleasantness Touch Awareness | |||||
|---|---|---|---|---|---|
| Palm [F(4,25) = 0.787, p = 0.544, R2 = 0.112] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | 0.14 | 0.21 | 0.05 | 0.65 | 0.52 |
| DCQ | 0.09 | 0.26 | 0.05 | 0.33 | 0.75 |
| Interoceptive Deficit (EDI-3) | 0.09 | 0.23 | 0.04 | 0.39 | 0.70 |
| Body Awareness (BPQ) | -0.31 | 0.21 | -0.04 | -1.47 | 0.15 |
| Face [F(4,25) = 1.204, p = 0.334, R2 = 0.161] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | -0.20 | 0.21 | -0.13 | -0.95 | 0.35 |
| DCQ | -0.27 | 0.25 | -0.31 | -1.04 | 0.31 |
| Interoceptive Deficit (EDI-3) | 0.38 | 0.23 | 0.33 | 1.69 | 0.10 |
| Body Awareness (BPQ) | -0.14 | 0.21 | -0.03 | -0.67 | 0.51 |
| Abdomen [F(4,25) = 0.407, p = 0.801, R2 = 0.061] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | -0.13 | 0.22 | -0.09 | -0.57 | 0.57 |
| DCQ | -0.22 | 0.27 | -0.30 | -0.83 | 0.41 |
| Interoceptive Deficit (EDI-3) | 0.19 | 0.24 | 0.18 | 0.78 | 0.44 |
| Body Awareness (BPQ) | -0.01 | 0.22 | -0.00 | -0.04 | 0.97 |
| Back [F(4,25) = 2.959, p = 0.039, R2 = 0.321] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | -0.05 | 0.19 | -0.01 | -0.24 | 0.81 |
| DCQ | -0.17 | 0.23 | -0.10 | -0.76 | 0.45 |
| Interoceptive Deficit (EDI-3) | 0.02 | 0.20 | 0.01 | 0.09 | 0.93 |
| Body Awareness (BPQ) | -0.46 | 0.19 | -0.05 | -2.47 | 0.02* |
| Forearm [F(4,25) = 3.770, p = 0.016, R2 = 0.376] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | 0.06 | 0.18 | 0.02 | 0.33 | 0.75 |
| DCQ | -0.62 | 0.22 | -0.38 | -2.82 | 0.01* |
| Interoceptive Deficit (EDI-3) | 0.51 | 0.20 | 0.23 | 2.61 | 0.01* |
| Body Awareness (BPQ) | -0.20 | 0.18 | -0.02 | -1.10 | 0.28 |
Notes: EDRC, Eating Disorder Risk Composite; EDI-3, Eating Disorder Inventory-3; BPQ, Body Perception Questionnaire.
* indicates p <0.05.
The linear multiple regression analyses calculated to predict overall touch intensity from symptoms of eating and dysmorphic concerns, emotional awareness and interoceptive sensibility scores were non-significant for all body sites [Palm: [F(4,25) = 0.652, p = 0.631, R2 = 0.094], Face [F(4,25) = 0.306, p = 0.871, R2 = 0.047], Abdomen [F(4,25) = 0.315, p = 0.865, R2 = 0.048], Back [F(4,25) = 0.755, p = 0.564, R2 = 0.108] and Forearm [F(4,25) = 0.953, p = 0.450, R2 = 0.132]. Full results are reported in Table 3.
Table 3. Unstandardized coefficients from linear simultaneous multiple regression models of eating and dysmorphic concerns and body awareness predictors of the overall touch intensity index at each body site (palm, face, abdomen, back and forearm).
| Overall Touch Intensity | |||||
|---|---|---|---|---|---|
| Palm [F(4,25) = 0.652, p = 0.631, R2 = 0.094] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | 0.19 | 0.22 | 0.06 | 0.86 | 0.40 |
| DCQ | 0.14 | 0.26 | 0.08 | 0.52 | 0.61 |
| Interoceptive Deficit (EDI-3) | 0.05 | 0.24 | 0.02 | 0.21 | 0.83 |
| Body Awareness (BPQ) | 0.00 | 0.22 | 0.00 | 0.01 | 0.99 |
| Face [F(4,25) = 0.306, p = 0.871, R2 = 0.047] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | 0.11 | 0.22 | 0.04 | 0.51 | 0.62 |
| DCQ | 0.18 | 0.27 | 0.11 | 0.65 | 0.52 |
| Interoceptive Deficit (EDI-3) | -0.03 | 0.24 | -0.01 | -0.11 | 0.91 |
| Body Awareness (BPQ) | -0.09 | 0.22 | -0.01 | -0.40 | 0.69 |
| Abdomen [F(4,25) = 0.315, p = 0.865, R2 = 0.048] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | -0.04 | 0.22 | -0.02 | -0.20 | 0.84 |
| DCQ | -0.08 | 0.27 | -0.05 | -0.28 | 0.78 |
| Interoceptive Deficit (EDI-3) | 0.26 | 0.24 | 0.13 | 1.06 | 0.30 |
| Body Awareness (BPQ) | -0.08 | 0.22 | -0.01 | -0.36 | 0.72 |
| Back [F(4,25) = 0.755, p = 0.564, R2 = 0.108] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | -0.15 | 0.22 | -0.04 | -0.68 | 0.50 |
| DCQ | 0.25 | 0.26 | 0.13 | 0.95 | 0.35 |
| Interoceptive Deficit (EDI-3) | 0.21 | 0.23 | 0.08 | 0.89 | 0.38 |
| Body Awareness (BPQ) | -0.14 | 0.21 | -0.01 | -0.63 | 0.53 |
| Forearm [F(4,25) = 0.953, p = 0.450, R2 = 0.132] | |||||
| B | SE | β | t-value | p-level | |
| EDRC (EDI-3) | -0.07 | 0.21 | -0.02 | -0.31 | 0.76 |
| DCQ | 0.28 | 0.26 | 0.16 | 1.07 | 0.30 |
| Interoceptive Deficit (EDI-3) | 0.20 | 0.23 | 0.09 | 0.88 | 0.38 |
| Body Awareness (BPQ) | -0.13 | 0.21 | -0.01 | -0.63 | 0.53 |
Notes: EDRC, Eating Disorder Risk Composite; EDI-3, Eating Disorder Inventory-3; BPQ, Body Perception Questionnaire.
Discussion
This study aimed to: a) investigate whether pleasantness and intensity ratings of touch vary across different body sites, b) explore associations between EDs and BDD traits with gentle touch applied at several body sites and in particular at emotionally salient body sites, i.e., abdomen and face; c) explore the relationship between self-reports of interoceptive sensibility and of emotional awareness with tactile experience at these varying body sites.
The results show that, as expected, perception of touch varied across skin sites according to both tactile pleasantness and intensity. Intensity ratings varied across the body sites examined, with touch to the back rated significantly less intense than any other body site. This is not surprisingly, considering that there is a strong relationship between a body region’s tactile innervation and individuals’ ability to spatially discriminate tactile stimuli [94], which characterises the back as a body site with relatively low innervation density and therefore low spatial acuity. Regarding tactile pleasantness, measured by means of hedonic ratings to three velocities of stroking, one CT-optimal (3 cm/s) and two non-CT optimal (0.3 cm/s and 30 cm/s), this was similar in profile across the body sites investigated. However, we found that there was a subtle difference in the ratings at the abdomen, so that participants rated this body area the least pleasant when touch was delivered at CT-optimal (3cm/s) velocity. However, this reduction in pleasantness for CT-optimal touch to the abdomen was not supported by a parallel increase in the intensity sensation experienced by participants at the same body site, given that overall, touch to the back but not to the abdomen was felt as the least intense. Contrary to our prediction, but in line with recent studies investigating whether perceived pleasantness of touch was modulated by subthreshold EDs psychopathology in two samples of healthy females [77, 78], we did not find evidence to support the idea of an association between preference for CT-optimal (3 cm/s) touch when applied to the abdomen and individual differences in any of the affective body image components. Interestingly however, exploratory analyses showed that perceived tactile preference for CT-optimal (3 cm/s) touch delivered to the back, was predicted by individual differences in body awareness (BPQ). Similarly, dysmorphic concerns and interoceptive deficits emerged as significant predictors for perceived pleasantness of CT-optimal (3 cm/s) touch to the forearm. We will now proceed to discuss specific findings in turn.
As expected, our main analysis of pleasantness ratings showed that participants expressed higher ratings for the CT-optimal (3 cm/s) velocity, compared to the slower (0.3 cm/s) or faster CT-non optimal (30 cm/s) stroking, at all body sites. This finding was corroborated by the regression analysis, which showed that a negative quadratic fit best described the stroking velocity-pleasantness profiles, giving the characteristic “inverted-U” shaped curves, at all body sites.
Essick et al. [52] found a similar preference for stroking at 5 cm/s on the cheek, compared to slower (0.5 cm/s) and faster (50 cm/s) strokes, and Essick et al. [3] showed that pleasantness was higher for stroking at 5 cm/s than at 20 cm/s at the forehead, finger (glabrous skin), thigh and calf. In partial agreement with current findings, we also found that (except for the abdomen area), pleasantness ratings at the glabrous, CT-free palm were not different from other body sites, where CTs are found. This was also supported by the result that a quadratic function best explained the data for relative touch pleasantness when applied to the glabrous palm.
Although numerous studies compare the CT-free palm to other hairy skin sites where CTs are present (see a recent review [12]), it is clear that pleasantness in touch can be experienced at glabrous skin sites, despite its reported lack of CTs [93, 95]. With this regard, and in accordance with our results, Löken et al. [3, 93] found that an ‘inverted-U’ velocity profile was present in pleasantness ratings for the palm, whist the overall mean was lower compared to stroking on the arm. One plausible explanation for these findings is that not only peripheral input, but also top-down mechanisms might have modulated the perception of pleasant touch at the glabrous palm [96, 97]. For instance, several studies have shown that touch stimulation of both hairy and glabrous skin similarly activates the orbitofrontal cortex [21–23], a brain area which is important for linking affective experiences to its rewarding value [97]. Therefore, as suggested by McGlone et al. [98], it may be plausible that the sensation of pleasantness at the palm is likely produced by the combination of unmyelinated and myelinated mechanoreceptive input, where higher-order cognitive evaluation of this type of touch is paramount in the judgment of its hedonic value.
In the present work and as predicted, we found a reduction in subjective reports of pleasantness when CT-optimal (3 cm/s) touch was delivered to the abdomen. Particularly, the abdomen was felt as less pleasant compared to all other body sites and was rated significantly less pleasant when touch was applied at the faster velocity (30 cm/s) compared to CT-optimal (3 cm/s) and slower (0.3 cm/s) touch. The effect was specific for the abdomen, given the significant differences observed when comparing the mean pleasantness ratings for CT-optimal touch to all other body sites. However, when we explored the relationship between pleasantness touch awareness (PTA) for the abdomen and individual differences in EDs and BDD traits, as well as body awareness/deficits no evidence for a linear association was found with such variables. This suggests that the reduction in perceived pleasantness when CT-optimal (3 cm/s) touch is delivered to this body area is not explained by participants’ EDs or BDD traits, nor by their hypersensitivity to bodily signals or interoceptive deficits. In keeping with previous findings [77, 78], our results may lend additional support that the observed reduced pleasantness of touch in anorexics patients, coupled with body-related anhedonia, might be a consequence rather than a predisposing factor of this disorder [33, 76].
Although we know from previous literature that the abdomen area has been identified as the body site women generally perceive as least attractive/satisfied with [59, 60, 99, 100], and that this body site is often subject to tactile overestimation in individuals suffering from EDs [55, 58], it might be possible that the body image measures included in the present study are somewhat limited in that they tap a global body dissatisfaction, rather than body site specific dissatisfaction. Therefore, more accurate measures of body dissatisfaction, for example, those obtained by administering the Body Areas Satisfaction Scale from the Multidimensional Body-Self Relations Questionnaire (MBSRQ-BASS) [101] or the Somatomap 3D which identifies perceptual distortions and dissatisfaction for specific body areas including the abdomen [59] might be more suitable for this scope in the future.
One alternative explanation to account for this overall reduction in perceived pleasantness at the abdomen may reside in the fact that for women the meaning of a touch is primarily influenced by how well the touch receiver knows the other, with touch from a same-sex person rated as unpleasant [102]. With this regard, a recent study from Suvilehto and colleagues [103] reported that across a broad range of European countries, whilst emotionally closer individuals in inner layers of the social network (e.g., family members) were allowed to touch wider body areas, touching by strangers was primarily limited to the hands and upper torso. However, the same study also reported that females, rather than males, evaluate touch as more pleasant and that they allow themselves to be touched on a larger body area than males (see also [104]). Furthermore, female same-sex touch was allowed without discomfort on most of the body’s surface [103]. Although, our sample of women do not rate CT-optimal (3 cm/s) touch applied to the abdomen as extremely unpleasant and evidence reported above seems to speak against this, at present we cannot rule out if this reduction in perceived pleasantness might be the result of a subjective feeling of intrusiveness of touch, as applied by the same-sex, stranger experimenter. This would also be compatible with the idea that higher BDD symptomology might lead to greater anxiety when being touched by a stranger, which in turn might reduce one’s rating of the experience as pleasant, a hypothesis which warrants further investigation.
An additional but not mutually exclusive explanation of the lack of association between reduced pleasantness at the abdomen and EDs/BDD traits may reside in the fact that in our sample there was essentially no evidence for particularly high-risk ED psychopathology, as shown by a relatively low spread and mean of EDRC (EDI-3) scores. Specifically, our sample of women scored an average of 18.53 on the EDRC, which is deemed to be ‘at low risk’ for the development of an ED [84]. We acknowledge this is a limitation to our study and that future research is necessary to better address whether clinical populations suffering from EDs and BDD may show reduced pleasantness of touch when delivered to emotionally salient body areas, such as the abdomen.
Interestingly, a further unexpected finding in our study may begin to elucidate a link between interoceptive affective touch and BDD traits. Indeed, our exploratory analyses showed that subjective ratings of pleasantness (as indexed by PTA) when delivered to the forearm were predicted by individual differences in dysmorphic appearance concerns, so that the higher women’s dysmorphic concerns the lower their preference for CT-optimal (3 cm/s) touch. Although, this result should be interpreted with caution due to the relatively small sample and the exploratory nature of the analyses conducted, this is the first investigation, to the best of our knowledge, to provide evidence of a relationship between a preference for CT-optimal (3 cm/s) touch and BDD symptomatology. To date, no studies so far have attempted to identify a link between BDD subthreshold symptomatology and pleasant touch. However, other studies have proposed that individuals with BDD may display lower levels of interoceptive awareness compared to individuals without this disorder [39]. Furthermore, research evidence suggests that a disconnection with the internal body may contribute to the misperception of and pre-occupation with features of the external body [47, 105]. Accordingly, our study provides first evidence that lower levels of dysmorphic appearance concerns may be linked to a preferential response to affective touch, similar to that observed by investigations into other disorders like AN, which commonly share symptoms of body image disturbance [32, 39].
Interestingly, we also found evidence that PTA (for the back and forearm) was predicted by self-reports of interoceptive sensibility (BPQ) and of emotional awareness (EDI-3 Interoceptive Deficit), respectively. These results resonate with previous findings from Cabrera et al. [88], who found that BPQ scores were elevated in participants with a self‐reported psychiatric diagnosis, as well as with previous clinical observations showing altered interoceptive functions across a range of psychiatric diagnoses, including Autism Spectrum Disorder, Post-Traumatic Stress Disorder [106], Anxiety [107, 108] and EDs [109].
With these regards, Pollatos et al. [36] proposed that individuals with higher levels of interoceptive awareness are likely to display heightened reactions to emotional stimuli, as they are more in tune with their bodily feedback. Furthermore, several authors have suggested that this increased awareness of somatic bodily sensations, and their subsequent appraisal as a threat to the self, contribute to the development and maintenance of anxiety disorders [107, 110]. Accordingly, we suggest that hyper-sensitivity to somatic bodily sensations (as measured by the BPQ), coupled with a greater sensitivity to CT-optimal touch may contribute all together to the aetiology and maintenance of those psychiatric conditions characterised by body image disturbance, a hypothesis that requires further exploration.
Contrary to our expectation that greater touch intensity would be associated with higher EDs and BDD traits, we did not find evidence of a linear association between overall touch intensity and self-reports of EDs and BDD. This finding speaks against that reported by Bischoff-Grethe et al. [39] according to which ill and recovered anorexics reported gentle touch as more intense, thus supporting a generalized finding of elevated intensity perception to somatosensory stimuli in both ill and weight-restored AN [111]. Although, the difference in our results compared to those obtained by Bischoff-Grethe et al. [39]’s study might be sampling-related (particularly with regards to the small sample and to the low variability in EDI-3 scores in our study), future research should aim to clarify whether a reduced responsivity to affective interoceptive stimuli coupled with an elevated intensity perception to somatosensory stimuli is linked to EDs and BDD traits. This would lend support to the idea that blunted interoception is linked to the (sensory) misperception of the body and its features, with both reported as being observed in both of these clinical conditions.
Furthermore, no linear associations between the overall touch intensity index and self-report measures of interoceptive sensibility and of emotional awareness were reported in this study. With these regards, although psychometric assessments of interoception might prove useful in investigating individual differences in state/trait measures of perceived sensibility across a range of internal bodily changes, they do not essentially address whether this subjective interoceptive sensibility relates to the strength of viscerosensory ‘input’ or corresponds to objective measures of accuracy on interoceptive tests [112]. Furthermore, it might be plausible that other interoceptive dimensions than those measured in the current study, might have a better concordance with the overall touch intensity index (see [73] for an extensive review on interoception taxonomy). For instance, an interesting study conducted by Garfinkel and colleagues [113], investigating whether processing of touch, compared to cardiac and respiratory signals are associated with different dimensions of interoception, including accuracy, sensibility (i.e., confidence) and meta-awareness (the latest defined as the metacognitive insight into one’s own interoceptive ability), reported that participants tended to have greater metacognitive insight into their touch performance, compared to respiratory metacognition. Furthermore, a correspondence between participants’ accuracy and confidence ratings was observed only for tactile signals, thus indicating that, subjective (perceived) and objective (actual) dimensions of interoceptive ability were aligned. Nonetheless, it should be noted that compared to our study, in the study by Garfinkel and colleagues [113] exteroceptive measures of touch were gathered by measuring participants’ sensitivity for touch (acuity) with an adapted version of the grating orientation task [114] and not by means of subjective ratings of intensity to CT-optimal and non-CT optimal touch. Future studies should aim at adopting a combined approach whereby measures of interoceptive accuracy, for example the heartbeat perception task [115], are coupled with a measure of subjective confidence in performing the task, but also more sophisticated analytic approaches that allow explicit quantification of how well confidence predicts accuracy within a given individual [113]. Additionally, more sophisticated interoceptive self-report scales like the Multidimensional Assessment of Interoceptive Awareness (MAIA), [116] might prove useful in capturing nuanced features of interoceptive experiences (e.g., the regulatory and accepting/non-judgmental aspects of interoceptive sensibility) that are relevant for clinical settings of EDs and BDD [117].
Our findings should also be viewed considering their limitations. First, in keeping with previous studies on affective touch in early adulthood and to ensure our sample is comparable in age to the vast majority of these studies (see [118], for a systematic review on affective touch studies over the lifetime), we similarly recruited participants with a mean age ranging from 18 to 40 years. Yet, we cannot rule out that age might have affected participants’ perceived pleasantness and intensity of touch. For instance, a study by Sehlstedt et al. [119] which evaluated the perceived pleasantness of a group of individuals aged 13–82 years, found that, for all ages, CT-targeted stimuli were rated as significantly more pleasant than stimuli delivered at CT-suboptimal velocities. Furthermore, the same study showed that pleasantness ratings to affective touch generally increase with age, suggesting that pleasant evaluation of touch follows an opposite trend from other tactile dimensions, such as intensity perception [119]. On the other hand, a brain imaging study by May et al. [120] investigating the effect of age on neural processing of pleasant soft touch stimuli to the palm and forearm, reported a different result. Despite not differing in their subjective reports of pleasantness of the soft touch, mature adults (29–55yrs) showed greater activation than young adult (20–28yrs) in the left dorsal anterior cingulate cortex (ACC) when anticipating the upcoming, rewarding soft touch. Furthermore, when considering age as a continuous variable, the authors reported older relative to younger individuals showed attenuated brain activation of the posterior insula during soft touch stimulation, suggesting that activation of this brain area declines with age. Future studies should better investigate (or control for) the effects of age on behavioural and neural processing of pleasant touch stimuli.
Second, it should be noted that the range of BMIs in the present sample was fairly wide (i.e., from normal-weight to obese with respect to the World Health Organization’s BMI classification scheme), with our sample of women reporting a mean BMI, which fell in the overweight category. Therefore, at present, we cannot rule out that the high variability observed in BMIs might have an impact on both women’s body image and subjective ratings of pleasant touch (and intensity) delivered at all body sites. The effects of BMI on several facets of body image [121], as well as on interoceptive processing [44, 122, 123] have indeed been recently described. Yet, the study by Crucianelli et al. [32] assessing the association between BMI and perception of affective touch in anorexics, compared to healthy controls, reported no significant correlations between the BMI and the pleasantness scores in each group, nor in the difference between slow and fast touch in each group. A further (brain imaging) study by Davidovic and colleagues [76] which asked anorexics and matched healthy controls to rate gentle skin strokes applied to the right forearm during fMRI scanning, similarly, reported no associations between pleasantness ratings and BMI. Given the heterogeneity of these results, future studies should better investigate the role of BMI in relation to cognitive processes underlying body image and subjective pleasantness responses to affective touch.
Finally, it is noteworthy that our sample was composed entirely by women, and as such our results cannot be generalised to a population of men. Previous studies reported that women respond more positively to touch than men [124] and they perceive affective touch and non-affective touch as more pleasant and more intense than men [125]. These findings are also corroborated by a recent meta-analysis by Russo, Ottaviani and Spitoni [126], which confirms this sex asymmetry in affective touch perception. Despite the fact that several factors may contribute to this sex asymmetry, including for e.g., genetic, psychological/cultural and hormonal (particularly testosterone levels in men) [127] factors, one might predict that an opposite pattern of results would be observed in this study, if men were to be included in the sample. That is men, compared to women, would rate CT-targeted touch as less pleasant compared to the two non-CT optimal stroking touches at all body sites and would also provide lower touch intensity ratings. Although we can only speculate on that, an important direction for future research could be to consider the role of gender when exploring affective touch in humans and include both males and females in studies sample.
Despite these limitations, this study provides evidence that overall, subjective experience of pleasant touch and intensity sensation varies across body sites, as we have already shown, but importantly, our findings begin to elucidate the unique associations between an increased sensitivity to affective interoceptive stimuli with body awareness and dysmorphic appearance concerns, thus proving the importance of extending research on affective touch to other clinical conditions underlying body image disturbance. These results may also have implications for future research looking to inform early interventions in body image disturbance, suggesting that addressing impaired processing of affective interoceptive stimuli may be the key to current treatment approaches available not only for AN but also for BDD populations. Along with alleviating symptoms of negative body image, future studies should also look at the relationship between the subjective experience of affective touch and positive body image, a multifaceted construct, distinct from negative body image, which is likely thought to be protective of physical health and foster psychological well-being [128].
Supporting information
(XLSX)
(XLSX)
Data Availability
All the relevant data are provided in anonymized datasets within the paper and its Supporting Information files.
Funding Statement
The authors received no specific funding for this work.
References
- 1.Morrison I, Löken LS, Olausson H. The skin as a social organ. Exp Brain Res. 2010; 204(3):305–14. doi: 10.1007/s00221-009-2007-y [DOI] [PubMed] [Google Scholar]
- 2.McGlone F, Vallbo AB, Olausson H, Loken L, Wessberg J. Discriminative touch and emotional touch. Can J Exp. 2007;61(3):173. doi: 10.1037/cjep2007019 [DOI] [PubMed] [Google Scholar]
- 3.Essick GK, McGlone F, Dancer C, Fabricant D, Ragin Y, et al. Quantitative assessment of pleasant touch. Neurosci Biobehav Rev. 2010;34(2):192–203. doi: 10.1016/j.neubiorev.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 4.Ackerley R, Carlsson I, Wester H, Olausson H, Backlund Wasling H. Touch perceptions across skin sites: differences between sensitivity, direction discrimination and pleasantness. Front Behav Neurosci. 2014;8, 54. doi: 10.3389/fnbeh.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ackerley R, Wasling HB, Liljencrantz J, Olausson H, Johnson RD, Wessberg J. Human C-tactile afferents are tuned to the temperature of a skin-stroking caress. J Neurosci. 2014;34(8):2879–2883. doi: 10.1523/JNEUROSCI.2847-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croy I, Geide H, Paulus M, Weidner K, Olausson H. Affective touch awareness in mental health and disease relates to autistic traits–An explorative neurophysiological investigation. Psychiatry Res. 2016;245:491–496. doi: 10.1016/j.psychres.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 7.Vallbo A, Olausson H, Wessberg J, Norrsell U. A system of unmyelinated afferents for innocuous mechanoreception in the human skin. Brain Res. 1993;628:301–304. doi: 10.1016/0006-8993(93)90968-s [DOI] [PubMed] [Google Scholar]
- 8.Vallbo AB, Olausson H, Wessberg J, Kakuda N. Receptive field characteristics of tactile units with myelinated afferents in hairy skin of human subjects. J Physiol. 1995;483(3):783–795. doi: 10.1113/jphysiol.1995.sp020622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vallbo ÅB, Olausson H, Wessberg J. Unmyelinated afferents constitute a second system coding tactile stimuli of the human hairy skin. J Physiol. 1999;81(6):2753–2763. doi: 10.1152/jn.1999.81.6.2753 [DOI] [PubMed] [Google Scholar]
- 10.Löken LS, Wessberg J, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nature neuroscience. 2009. May;12(5):547. doi: 10.1038/nn.2312 [DOI] [PubMed] [Google Scholar]
- 11.Croy I, Luong A, Triscoli C, Hofmann E, Olausson H, Sailer U. Interpersonal stroking touch is targeted to C tactile afferent activation. Behav Brain Res. 2016;1(297):37–40. [DOI] [PubMed] [Google Scholar]
- 12.Morrison I. ALE meta‐analysis reveals dissociable networks for affective and discriminative aspects of touch. Human brain mapping. 2016;37(4):1308–1320. doi: 10.1002/hbm.23103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Dev Sci. 2010;13(2):271–8. doi: 10.1111/j.1467-7687.2009.00890.x [DOI] [PubMed] [Google Scholar]
- 14.Fairhurst MT, Löken L, Grossmann T. Physiological and behavioral responses reveal 9-month-old infants’ sensitivity to pleasant touch. Psychol Sci. 2014;25(5):1124–31. doi: 10.1177/0956797614527114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walker SC, McGlone FP. The social brain: neurobiological basis of affiliative behaviours and psychological well-being. Neuropeptides. 2013;47(6): 379–393. doi: 10.1016/j.npep.2013.10.008 [DOI] [PubMed] [Google Scholar]
- 16.von Mohr M, Kirsch LP, Fotopoulou A. The soothing function of touch: affective touch reduces feelings of social exclusion. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morrison I, Björnsdotter M, Olausson H. Vicarious responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. J Neurosci: The Official Journal of the Society for Neuroscience. 2011;31(26):9554–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kirsch LP, Besharati S, Papadaki C, Crucianelli L, Bertagnoli S, et al. Damage to the right insula disrupts the perception of affective touch. Elife. 2020;9. doi: 10.7554/eLife.47895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olausson H, Lamarre Y, Backlund H, Morin C, Wallin BG, et al. Unmyelinated tactile afferents signal touch and project to insular cortex. Nat Neurosci. 2002;5(9):900–4. doi: 10.1038/nn896 [DOI] [PubMed] [Google Scholar]
- 20.Olausson HW, Cole J, Vallbo Å, McGlone F, Elam M, et al. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci Letters. 2008;436(2):128–132. doi: 10.1016/j.neulet.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 21.McGlone F, Olausson H, Boyle JA, Jones‐Gotman M, Dancer C, Guest S, et al. Touching and feeling: differences in pleasant touch processing between glabrous and hairy skin in humans. Eur J Neurosci. 2012;35(11):1782–1788. doi: 10.1111/j.1460-9568.2012.08092.x [DOI] [PubMed] [Google Scholar]
- 22.Francis S, Rolls ET, Bowtell R, Mcglone F, O’Doherty J, et al. The representation of pleasant touch in the brain and its relationship with taste and olfactory areas. Neuroreport. 1999;10(3):453–459. doi: 10.1097/00001756-199902250-00003 [DOI] [PubMed] [Google Scholar]
- 23.Rolls ET, O’Doherty J, Kringelbach ML, Francis S, Bowtell R, McGlone F. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb Cortex. 2003;13(3):308–317. doi: 10.1093/cercor/13.3.308 [DOI] [PubMed] [Google Scholar]
- 24.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci. 2002;3(8):655–666. [DOI] [PubMed] [Google Scholar]
- 25.Craig AD. How do you feel—now? The anterior insula and human awareness. Nature Rev Neurosci. 2009;10(1). [DOI] [PubMed] [Google Scholar]
- 26.Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176 [DOI] [PubMed] [Google Scholar]
- 27.Crucianelli L, Krahé C, Jenkinson PM, Fotopoulou AK. Interoceptive ingredients of body ownership: Affective touch and cardiac awareness in the rubber hand illusion. Cortex. 2018;104:180–192. doi: 10.1016/j.cortex.2017.04.018 [DOI] [PubMed] [Google Scholar]
- 28.Croy I, Bierling A, Sailer U, Ackerley R. Individual variability of pleasantness ratings to stroking touch over different velocities. Neurosci. 2020. doi: 10.3390/neurosci1020006 [DOI] [PubMed] [Google Scholar]
- 29.Harjunen VJ, Spapé M, Ahmed I, Jacucci G, Ravaja N. Individual differences in affective touch: Behavioral inhibition and gender define how an interpersonal touch is perceived. Pers Individ Differ. 2017;107:88–95. [Google Scholar]
- 30.Voos AC, Pelphrey K, Kaiser MD. Autistic traits are associated with diminished neural response to affective touch. SCAN. 2013;8(4):378–86. doi: 10.1093/scan/nss009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krahé C, Drabek MM, Paloyelis Y, Fotopoulou A. Affective touch and attachment style modulate pain: a laser-evoked potentials study. Philos Trans R Soc Lond B Biol Sci. 2016;371(1708)20160009. doi: 10.1098/rstb.2016.0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crucianelli L, Cardi V, Treasure J, Jenkinson PM, Fotopoulou A. The perception of affective touch in anorexia nervosa. Psychiatry Res. 2016;239:72–78. doi: 10.1016/j.psychres.2016.01.078 [DOI] [PubMed] [Google Scholar]
- 33.Crucianelli L, Demartini B, Goeta D, Nisticò V, Saramandi A, et al. The anticipation and perception of affective touch in women with and recovered from Anorexia Nervosa. Neurosci. 2020. doi: 10.3390/neurosci1020006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tiggemann M. Body image across the adult life span: Stability and change. Body image. 2004. Jan 1;1(1):29–41. doi: 10.1016/S1740-1445(03)00002-0 [DOI] [PubMed] [Google Scholar]
- 35.Cash TF, Deagle III EA The nature and extent of body‐image disturbances in anorexia nervosa and bulimia nervosa: A meta‐analysis. Int J Eat Disord. 1997;22(2):107–126. [PubMed] [Google Scholar]
- 36.Pollatos O, Kurz AL, Albrecht J, Schreder T, Kleemann AM, Schöpf V, et al. Reduced perception of bodily signals in anorexia nervosa. Eat Behav. 2008;9(4):381–388. doi: 10.1016/j.eatbeh.2008.02.001 [DOI] [PubMed] [Google Scholar]
- 37.Khalsa SS, Craske MG, Li W, Vangala S, Strober M, Feusner JD. Altered interoceptive awareness in anorexia nervosa: effects of meal anticipation, consumption and bodily arousal. Int J Eat Disord. 2015; 48(7):889–897. doi: 10.1002/eat.22387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Badoud D, Tsakiris M. From the body’s viscera to the body’s image: Is there a link between interoception and body image concerns?. Neuroscience & Biobehavioral Reviews. 2017. Jun 1;77:237–46. [DOI] [PubMed] [Google Scholar]
- 39.Bischoff-Grethe A, Wierenga CE, Berner LA, Simmons AN, Bailer U, Paulus MP, et al. Neural hypersensitivity to pleasant touch in women remitted from anorexia nervosa. Transl Psychiatry. 2018;8(1):1–13. doi: 10.1038/s41398-017-0025-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mehling WE, Gopisetty V, Daubenmier J, Price C J, Hecht FM, Stewart A. Body awareness: construct and self-report measures. PloS one. 2009;4(5)e5614. doi: 10.1371/journal.pone.0005614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arciero G. & Guidano V. F. Experience, explanation, and the quest for coherence. In Constructions of Disorder: Meaning-Making Frameworks for Psychotherapy (eds Neimeyer R. A. & Raskin J. D.) 91–118 (American Psychological Association, Washington, DC, US, 2000). [Google Scholar]
- 42.Eshkevari E, Rieger E, Musiat P, Treasure J. An investigation of interoceptive sensitivity in eating disorders using a heartbeat detection task and a self‐report measure. European Eating Disorders Review. 2014. Sep;22(5):383–8. [DOI] [PubMed] [Google Scholar]
- 43.Martin E, Dourish CT, Rotshtein P, Spetter MS, Higgs S. Interoception and disordered eating: A systematic review. Neuroscience & Biobehavioral Reviews. 2019. Dec 1;107:166–91. doi: 10.1016/j.neubiorev.2019.08.020 [DOI] [PubMed] [Google Scholar]
- 44.Todd J, Aspell JE, Barron D, Swami V. Multiple dimensions of interoceptive awareness are associated with facets of body image in British adults. Body Image. 2019. Jun 1;29:6–16. doi: 10.1016/j.bodyim.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 45.Ainley V, Tsakiris M. Body conscious? Interoceptive awareness, measured by heartbeat perception, is negatively correlated with self-objectification. PloS one. 2013;8(2)e55568. doi: 10.1371/journal.pone.0055568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kunstman JW, Clerkin EM, Palmer K, Peters MT, Dodd DR, Smith AR. The power within: The experimental manipulation of power interacts with trait BDD symptoms to predict interoceptive accuracy. J Behav Ther Exp Psychiatry. 2016;50,178–186. doi: 10.1016/j.jbtep.2015.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaplan RA, Enticott PG, Hohwy J, Castle DJ, Rossell SL. Is body dysmorphic disorder associated with abnormal bodily self-awareness? A study using the rubber hand illusion. PLoS One;2014. 9(6). doi: 10.1371/journal.pone.0099981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Diagnostic and Statistical Manual of Mental Disorders: Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association, 2013. [Google Scholar]
- 49.Kaplan RA, Rossell SL, Enticott PG, Castle DJ. Own-body perception in body dysmorphic disorder. Cogn Neuropsychiatry. 2013;18(6):594–614. doi: 10.1080/13546805.2012.758878 [DOI] [PubMed] [Google Scholar]
- 50.Liu Q, Vrontou S, Rice FL, Zylka MJ, Dong X, Anderson DJ. Molecular genetic visualization of a rare subset of unmyelinated sensory neurons that may detect gentle touch. Nat Neurosci. 2007;10(8):946–948. doi: 10.1038/nn1937 [DOI] [PubMed] [Google Scholar]
- 51.Walker SC, Trotter PD, Woods A, McGlone F. Vicarious ratings of social touch reflect the anatomical distribution and velocity tuning of C-tactile afferents: A hedonic homunculus?. Behav Brain Res. 2017;320: 91–96. doi: 10.1016/j.bbr.2016.11.046 [DOI] [PubMed] [Google Scholar]
- 52.Essick GK, James A, McGlone FP. Psychophysical assessment of the affective components of non-painful touch. Neuroreport. 1999;10(10):2083–2087. doi: 10.1097/00001756-199907130-00017 [DOI] [PubMed] [Google Scholar]
- 53.Kandel ER, Schawartz JH, Jessell TM. Principles of neural science. New York: McGraw-Hill Companies. 2000. doi: 10.1126/science.287.5451.273 [DOI] [Google Scholar]
- 54.Mountcastle VB. The Sensory Hand: Neural mechanisms of somatic sensation. Cambridge, MA, US, University Press. 2005. doi: 10.1016/j.neures.2005.10.004 [DOI] [Google Scholar]
- 55.Keizer A, Smeets MAM, Dijkerman HC, Van den Hout M, Klugkist I, Van Elburg A, et al. Tactile body image disturbance in anorexia nervosa. Psychiatry Res. 2011;190(1):115–120. doi: 10.1016/j.psychres.2011.04.031 [DOI] [PubMed] [Google Scholar]
- 56.Keizer A, Smeets MAM, Dijkerman HC, van Elburg A, Postma A. Aberrant somatosensory perception in Anorexia Nervosa. Psychiatry Res. 2012;200(2–3):530–537. doi: 10.1016/j.psychres.2012.05.001 [DOI] [PubMed] [Google Scholar]
- 57.Keizer A, van Elburg A, Helms R, Dijkerman HC. A virtual reality full body illusion improves body image disturbance in anorexia nervosa. PloS one. 2016;11(10):e0163921. doi: 10.1371/journal.pone.0163921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Spitoni GF, Serino A, Cotugno A, Mancini F, Antonucci G, Pizzamiglio L. The two dimensions of the body representation in women suffering from Anorexia Nervosa. Psychiatry Res. 2015;230(2):181–188. doi: 10.1016/j.psychres.2015.08.036 [DOI] [PubMed] [Google Scholar]
- 59.Ralph-Nearman C, Arevian AC, Puhl M, Kumar R, Villaroman D, Suthana N et al. A Novel Mobile Tool (Somatomap) to Assess Body Image Perception Pilot Tested With Fashion Models and Nonmodels: Cross-Sectional Study. JMIR mental health. 2019;6(10), e14115. doi: 10.2196/14115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bellard AM, Cornelissen PL, Mian E, Cazzato V. The ageing body: contributing attitudinal factors towards perceptual body size estimates in younger and middle-aged women. Arch Womens Ment Health. 2020;1–13. doi: 10.1007/s00737-020-01046-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Skałacka K, Pajestka G. “No matter how old I am, I don’t like what my stomach looks like.” Lifespan perspective on the changes in self-assessment of attractiveness and life satisfaction in women. J Women Aging. 2020;1–9. [DOI] [PubMed] [Google Scholar]
- 62.Lewis‐Smith H, Diedrichs PC, Rumsey N, Harcourt D, A systematic review of interventions on body image and disordered eating outcomes among women in midlife. Int J Eat Disord. 2016;49(1):5–18. doi: 10.1002/eat.22480 [DOI] [PubMed] [Google Scholar]
- 63.Kollei I, Schieber K, de Zwaan M, Svitak M, Martin A. Body dysmorphic disorder and nonweight‐related body image concerns in individuals with eating disorders. Int J Eat Disord. 2013;46(1):52–59. doi: 10.1002/eat.22067 [DOI] [PubMed] [Google Scholar]
- 64.Jansen A, Nederkoorn C, Mulkens S. Selective visual attention for ugly and beautiful body parts in eating disorders. Behav Res Ther. 2005;43(2):183–196. doi: 10.1016/j.brat.2004.01.003 [DOI] [PubMed] [Google Scholar]
- 65.Jansen A, Smeets T, Martijn C, Nederkoorn C. I see what you see: The lack of a self‐serving body‐image bias in eating disorders. Br J Clin Psychol. 2006;45(1):123–135. doi: 10.1348/014466505X50167 [DOI] [PubMed] [Google Scholar]
- 66.Haxby JV, Hoffman EA, Gobbini MI. The distributed human neural system for face perception. TiCS. 2000;4(6):223–233. [DOI] [PubMed] [Google Scholar]
- 67.Phillips KA, McElroy SL, Keck PE. Pope HG, Hudson JI. Body dysmorphic disorder: 30 cases of imagined ugliness. Am J Psychiatry. 1993;150:302–302. doi: 10.1176/ajp.150.2.302 [DOI] [PubMed] [Google Scholar]
- 68.Phillips KA, Menard W, Fay C. Gender similarities and differences in 200 individuals with body dysmorphic disorder. Compr Psychiatry. 2006;47(2):77–87. doi: 10.1016/j.comppsych.2005.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phillips KA, Pagano ME, Menard W, Fay C, Stout RL. Predictors of remission from body dysmorphic disorder: a prospective study. J Nerv Ment Dis. 2005;193(8)564. doi: 10.1097/01.nmd.0000172681.51661.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Porges S. Body Perception Questionnaire. Laboratory of Developmental Assessment, University of Maryland. 1993. [Google Scholar]
- 71.Garner DM. Eating disorder inventory-3 (EDI-3). Professional manual. Odessa, FL: Psychological Assessment Resources. 2004. [Google Scholar]
- 72.Eshkevari E, Rieger E, Longo MR, Haggard P, Treasure J. Persistent body image disturbance following recovery from eating disorders. Int J Eat Disord. 2014;47(4): 400–409. doi: 10.1002/eat.22219 [DOI] [PubMed] [Google Scholar]
- 73.Khalsa SS, Adolphs R, Cameron OG, Critchley HD, Davenport PW, Feinstein JS, et al. Interoception and mental health: a roadmap. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2018. Jun 1;3(6):501–13. doi: 10.1016/j.bpsc.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jönsson EH, Wasling HB, Wagnbeck V, Dimitriadis M, Georgiadis JR, Olausson H, et al. Unmyelinated tactile cutaneous nerves signal erotic sensations. J Sex Med. 2015;12(6)1338–1345. doi: 10.1111/jsm.12905 [DOI] [PubMed] [Google Scholar]
- 75.Luong A, Bendas J, Etzi R, Olausson H, Croy I. The individual preferred velocity of stroking touch as a stable measurement. Physiol Behav. 2017;177, 129–134. doi: 10.1016/j.physbeh.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 76.Davidovic M, Karjalainen L, Starck G, Wentz E, Björnsdotter M, Olausson H. Abnormal brain processing of gentle touch in Anorexia Nervosa. Psychiatry Res. Neuroimaging. 2018;281,53–60. doi: 10.1016/j.pscychresns.2018.08.007 [DOI] [PubMed] [Google Scholar]
- 77.Carey M, Crucianelli L, Preston C, Fotopoulou A. The effect of visual capture towards subjective embodiment within the full body illusion. Scientific reports. 2019. Feb 27;9(1):1–2. doi: 10.1038/s41598-018-37186-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Carey M, Crucianelli L, Preston C, Fotopoulou A. The role of affective touch in whole-body embodiment remains equivocal. Consciousness and Cognition. 2021. Jan 1;87:103059. doi: 10.1016/j.concog.2020.103059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146 [DOI] [PubMed] [Google Scholar]
- 80.Cazzato V, Mian E, Serino A, Mele S, Urgesi C. Distinct contributions of extrastriate body area and temporoparietal junction in perceiving one’s own and others’ body. CANB. 2015;15(1):211–228. doi: 10.3758/s13415-014-0312-9 [DOI] [PubMed] [Google Scholar]
- 81.Cazzato V, Mian E, Mele S, Tognana G, Todisco P, Urgesi C. The effects of body exposure on self-body image and esthetic appreciation in anorexia nervosa. Exp Brain Res. 2016;234(3):695–709. doi: 10.1007/s00221-015-4498-z [DOI] [PubMed] [Google Scholar]
- 82.Stanford SC, Lemberg R. A clinical comparison of men and women on the Eating Disorder Inventory-3 (EDI-3) and the Eating Disorder Assessment for Men (EDAM). Eat Disorders. 2012;20(5):379–394. [DOI] [PubMed] [Google Scholar]
- 83.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4 [DOI] [PubMed] [Google Scholar]
- 84.Clausen L, Rosenvinge JH, Friborg O, Rokkedal K. Validating the Eating Disorder Inventory-3 (EDI-3): A comparison between 561 female eating disorders patients and 878 females from the general population. J Psychopathol Behav Assess. 2011;33(1): 101–110. doi: 10.1007/s10862-010-9207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Oosthuizen P, Lambert T, Castle DJ. Dysmorphic concern: prevalence and associations with clinical variables. Aust N Z J Psychiatry. 1998;32(1):129–132. doi: 10.3109/00048679809062719 [DOI] [PubMed] [Google Scholar]
- 86.Mancuso SG, Knoesen NP, Castle DJ. The Dysmorphic Concern Questionnaire: A screening measure for body dysmorphic disorder. Aust N Z J Psychiatry. 2010;44: 535–542. doi: 10.3109/00048671003596055 [DOI] [PubMed] [Google Scholar]
- 87.Jorgensen L, Castle D, Roberts C, Groth-Marnat G. A clinical validation of the Dysmorphic Concern Questionnaire. Aust N Z J Psychiatry. 2001;35(1):124–8. doi: 10.1046/j.1440-1614.2001.00860.x [DOI] [PubMed] [Google Scholar]
- 88.Cabrera A, Kolacz J, Pailhez G, Bulbena‐Cabre A, Bulbena A, Porges SW. Assessing body awareness and autonomic reactivity: Factor structure and psychometric properties of the Body Perception Questionnaire‐Short Form (BPQ‐SF ). Int J Methods Psychiatr. 2018;27(2):e1596. doi: 10.1002/mpr.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peirce JW. PsychoPy—psychophysics software in Python. J Neurosci Methods. 2007;162(1–2):8–13. doi: 10.1016/j.jneumeth.2006.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crucianelli L, Metcalf NK, Fotopoulou AK, Jenkinson PM. Bodily pleasure matters: velocity of touch modulates body ownership during the rubber hand illusion. Front Psychol. 2013;4,703. doi: 10.3389/fpsyg.2013.00703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pawling R, Cannon PR, McGlone FP, Walker SC. C-tactile afferent stimulating touch carries a positive affective value. PloS one. 2017;12(3). doi: 10.1371/journal.pone.0173457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Haggarty CJ, Malinowski P, McGlone FP, Walker SC. Autistic traits modulate cortical responses to affective but not discriminative touch. Eur J Neurosci. 2020;51(8):1844–55. doi: 10.1111/ejn.14637 [DOI] [PubMed] [Google Scholar]
- 93.Löken LS, Evert M, Wessberg J. Pleasantness of touch in human glabrous and hairy skin: order effects on affective ratings. Brain Res. 2011;12:1417:9–15. doi: 10.1016/j.brainres.2011.08.011 [DOI] [PubMed] [Google Scholar]
- 94.Corniani G, Saal HP. Tactile innervation densities across the whole body. Journal of Neurophysiology. 2020. Oct 1;124(4):1229–40. doi: 10.1152/jn.00313.2020 [DOI] [PubMed] [Google Scholar]
- 95.Klöcker A, Wiertlewski M, Théate V, Hayward V, Thonnard JL. Physical factors influencing pleasant touch during tactile exploration. Plos one. 2013;8(11)e79085. doi: 10.1371/journal.pone.0079085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Berridge KC, Kringelbach ML. Affective neuroscience of pleasure: reward in humans and animals. Psychopharmacology. 2008;199(3):457–480. doi: 10.1007/s00213-008-1099-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rolls ET. The affective and cognitive processing of touch, oral texture, and temperature in the brain. Neurosci Biobehav Rev. 2010;34(2):237–245. doi: 10.1016/j.neubiorev.2008.03.010 [DOI] [PubMed] [Google Scholar]
- 98.McGlone F, Wessberg J, Olausson H. Discriminative and affective touch: sensing and feeling. Neuron. 2014;82(4):737–755. doi: 10.1016/j.neuron.2014.05.001 [DOI] [PubMed] [Google Scholar]
- 99.Bauer A, Schneider S, Waldorf M, Braks K, Huber TJ, et al. Selective visual attention towards oneself and associated state body satisfaction: an eye-tracking study in adolescents with different types of eating disorders. J Abnorm Child Psychol. 2017;45(8):1647–1661. doi: 10.1007/s10802-017-0263-z [DOI] [PubMed] [Google Scholar]
- 100.Horndasch S, Kratz O, Holczinger A, Heinrich H, Hönig F, Nöth E, et al. “Looks do matter”–visual attentional biases in adolescent girls with eating disorders viewing body images. Psychiatry Res, 2012;198, 321–323. doi: 10.1016/j.psychres.2011.12.029 [DOI] [PubMed] [Google Scholar]
- 101.Cash TF. The multidimensional body-self relations questionnaire users’ manual. Available from the author at www.body-images.com. 2000. [Google Scholar]
- 102.Heslin R, Nguyen TD, Nguyen ML. Meaning of touch: The case of touch from a stranger or same sex person. J Nonverbal Behav. 1983;7(3):147–157. [Google Scholar]
- 103.Suvilehto JT, Glerean E, Dunbar RI, Hari R, Nummenmaa L. Topography of social touching depends on emotional bonds between humans. PNAS. 2015;112(45):13811–13816. doi: 10.1073/pnas.1519231112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jourard SM. An Exploratory Study of Body‐Accessibility. Br J Clin Psychol. 1966;5(3):221–231. doi: 10.1111/j.2044-8260.1966.tb00978.x [DOI] [PubMed] [Google Scholar]
- 105.Veale D. Advances in a cognitive behavioural model of body dysmorphic disorder. Body image. 2004;1(1):113–125. doi: 10.1016/S1740-1445(03)00009-3 [DOI] [PubMed] [Google Scholar]
- 106.Harshaw C. Interoceptive dysfunction: Toward an integrated framework for understanding somatic and affective disturbance in depression. Psychol Bull. 2015;141(2):311. doi: 10.1037/a0038101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Domschke K, Stevens S, Pfleiderer B, Gerlach AL. Interoceptive sensitivity in anxiety and anxiety disorders: an overview and integration of neurobiological findings. Clin Psychol Rev. 2010;30(1):1–11. doi: 10.1016/j.cpr.2009.08.008 [DOI] [PubMed] [Google Scholar]
- 108.Mallorquí-Bagué N, Bulbena A, Pailhez G, Garfinkel SN, Critchley HD. Mind-body interactions in anxiety and somatic symptoms. Harv Rev Psychiatry. 2016;24(1):53–60. doi: 10.1097/HRP.0000000000000085 [DOI] [PubMed] [Google Scholar]
- 109.Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nat Rev Neurosci. 2009;10 (8):573. doi: 10.1038/nrn2682 [DOI] [PubMed] [Google Scholar]
- 110.Ehlers A, Breuer P, Dohn D, Fiegenbaum W. Heartbeat perception and panic disorder: possible explanations for discrepant findings. Behav Res Ther. 1995;33(1):69–76. doi: 10.1016/0005-7967(94)e0002-z [DOI] [PubMed] [Google Scholar]
- 111.Brand‐Gothelf A, Parush S, Eitan Y, Admoni S, Gur E, Stein D. Sensory modulation disorder symptoms in anorexia nervosa and bulimia nervosa: A pilot study. Int J Eat Disord. 2016;49(1):59–68. doi: 10.1002/eat.22460 [DOI] [PubMed] [Google Scholar]
- 112.Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: distinguishing interoceptive accuracy from interoceptive awareness. Biological psychology. 2015. Jan 1;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 113.Garfinkel SN, Manassei MF, Hamilton-Fletcher G, In den Bosch Y, Critchley HD, Engels M. Interoceptive dimensions across cardiac and respiratory axes. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016. Nov 19;371(1708):20160014. doi: 10.1098/rstb.2016.0014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van Boven RW, Johnson KO. The limit of tactile spatial resolution in humans: grating orientation discrimination at the lip, tongue, and finger. Neurology. 1994. Dec 1;44(12):2361. doi: 10.1212/wnl.44.12.2361 [DOI] [PubMed] [Google Scholar]
- 115.Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981. Jul;18(4):483–8. doi: 10.1111/j.1469-8986.1981.tb02486.x [DOI] [PubMed] [Google Scholar]
- 116.Mehling WE, Price C, Daubenmier JJ, Acree M, Bartmess E, Stewart A. The multidimensional assessment of interoceptive awareness (MAIA). PloS one. 2012. Nov 1;7(11):e48230. doi: 10.1371/journal.pone.0048230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mehling W. Differentiating attention styles and regulatory aspects of self-reported interoceptive sensibility. Philosophical Transactions of the Royal Society B: Biological Sciences. 2016. Nov 19;371(1708):20160013. doi: 10.1098/rstb.2016.0013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cruciani G, Zanini L, Russo V, Mirabella M, Palamoutsi EM, Spitoni GF. Strengths and weaknesses of affective touch studies over the lifetime: A systematic review. Neuroscience & Biobehavioral Reviews. 2021. Apr 20. doi: 10.1016/j.neubiorev.2021.04.012 [DOI] [PubMed] [Google Scholar]
- 119.Sehlstedt I, Ignell H, Backlund Wasling H, Ackerley R, Olausson H, Croy I. Gentle touch perception across the lifespan. Psychology and aging. 2016. Mar;31(2):176. doi: 10.1037/pag0000074 [DOI] [PubMed] [Google Scholar]
- 120.May AC, Stewart JL, Paulus MP, Tapert SF. The effect of age on neural processing of pleasant soft touch stimuli. Frontiers in behavioral neuroscience. 2014. Feb 21;8:52. doi: 10.3389/fnbeh.2014.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Swami V, Frederick DA, Aavik T, Alcalay L, Allik J, Anderson D, et al. The attractive female body weight and female body dissatisfaction in 26 countries across 10 world regions: Results of the International Body Project I. Personality and social psychology bulletin. 2010. Mar;36(3):309–25. doi: 10.1177/0146167209359702 [DOI] [PubMed] [Google Scholar]
- 122.Cameron OG. Interoception: the inside story—a model for psychosomatic processes. Psychosomatic medicine. 2001. Sep 1;63(5):697–710. doi: 10.1097/00006842-200109000-00001 [DOI] [PubMed] [Google Scholar]
- 123.Herbert BM, Pollatos O. Attenuated interoceptive sensitivity in overweight and obese individuals. Eating behaviors. 2014. Aug 1;15(3):445–8. doi: 10.1016/j.eatbeh.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 124.Stier DS, Hall JA. Gender differences in touch: An empirical and theoretical review. Journal of personality and social psychology. 1984. Aug;47(2):440. [Google Scholar]
- 125.Jönsson EH, Bendas J, Weidner K, Wessberg J, Olausson H, Wasling HB, et al. The relation between human hair follicle density and touch perception. Scientific reports. 2017. May 31;7(1):1–0. doi: 10.1038/s41598-016-0028-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Russo V, Ottaviani C, Spitoni GF. Affective touch: A meta-analysis on sex differences. Neuroscience & Biobehavioral Reviews. 2020. Jan 1;108:445–52. doi: 10.1016/j.neubiorev.2019.09.037 [DOI] [PubMed] [Google Scholar]
- 127.Burris AS, Gracely RH, Carter CS, Sherins RJ, Davidson JM. Testosterone therapy is associated with reduced tactile sensitivity in human males. Hormones and behavior. 1991. Jun 1;25(2):195–205. doi: 10.1016/0018-506x(91)90050-r [DOI] [PubMed] [Google Scholar]
- 128.Tylka TL, Wood-Barcalow NL. What is and what is not positive body image? Conceptual foundations and construct definition. Body image. 2015. Jun 1;14:118–29. doi: 10.1016/j.bodyim.2015.04.001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
(XLSX)
Data Availability Statement
All the relevant data are provided in anonymized datasets within the paper and its Supporting Information files.