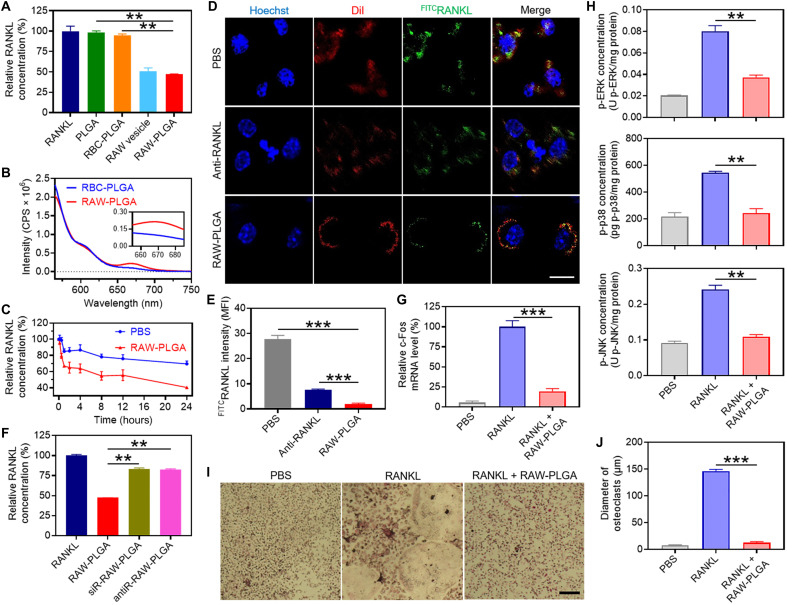
Fig. 3. In vitro RANKL-scavenging and anti-osteoclastogenesis efficiencies of RAW-PLGA nanodecoys.
(A) Relative RANKL concentration after incubation with PLGA NPs, RBC-PLGA NPs, RAW vesicles, or RAW-PLGA nanodecoys at the initial RANKL concentration of 200 pg/ml (n = 3). (B) FRET analysis of Cy5RAW-PLGA nanodecoys (or Cy5RBC-PLGA NPs) and Cy3RANKL (λex = 550 nm). (C) Extracellular RANKL concentration of RAW 264.7 cells after incubation with PBS or RAW-PLGA nanodecoys for different times at the initial RANKL concentration of 100 ng/ml (n = 3). (D) CLSM images of RAW 264.7 cells after 4-hour incubation with PBS, RAW-PLGA nanodecoys, or anti-RANKL in the presence of FITCRANKL (scale bar, 10 μm). Cell nuclei and membrane were stained with Hoechst 33258 and DiI, respectively. (E) Quantified mean fluorescence intensity of FITCRANKL in RAW 264.7 cells in (D) (n = 3). (F) RANKL-scavenging efficiencies of RAW-PLGA nanodecoys, antiR-RAW-PLGA nanodecoys, and siR-RAW-PLGA nanodecoys (n = 3). Relative c-Fos mRNA level (G) and p-ERK, p-p38, and p-JNK levels (H) in BMMs (n = 3). (I) TRAP staining images of BMM-derived osteoclasts (scale bar, 100 μm). (J) Size of TRAP-positive multinucleated osteoclasts (≥3 nuclei) (n = 3). In (G) to (J), cells were treated with RAW-PLGA nanodecoys (100 μg PLGA/ml) or PBS for 24 hours in the presence of RANKL (100 ng/ml), and cells treated with PBS served as the control.