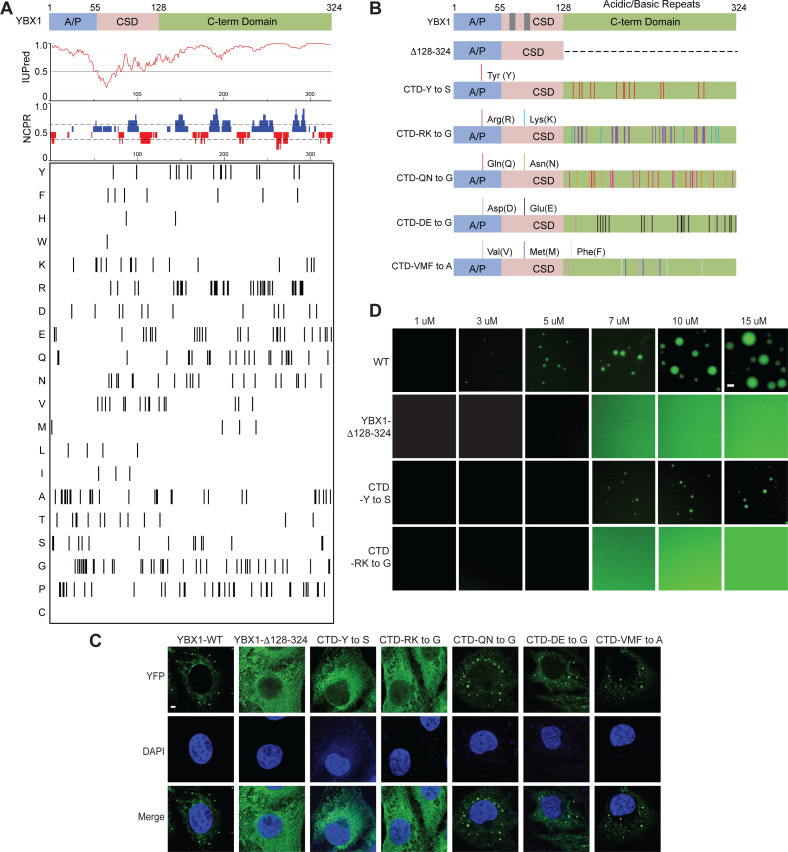
Figure 3. YBX1 phase separation is governed by association of aromatic and basic amino acids in C-terminal IDR.
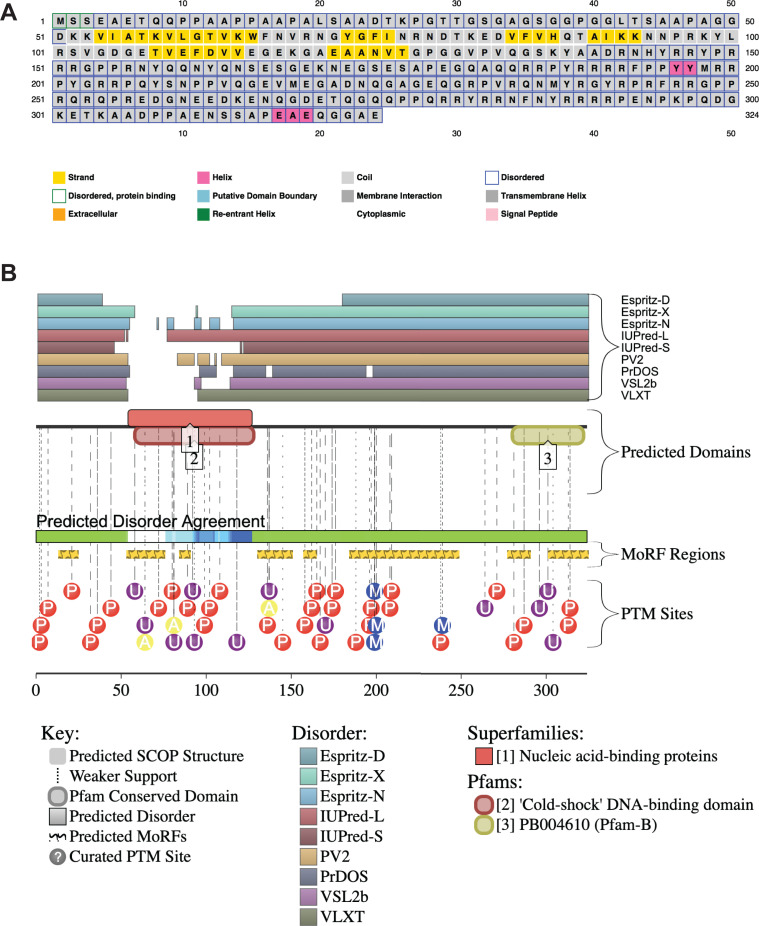
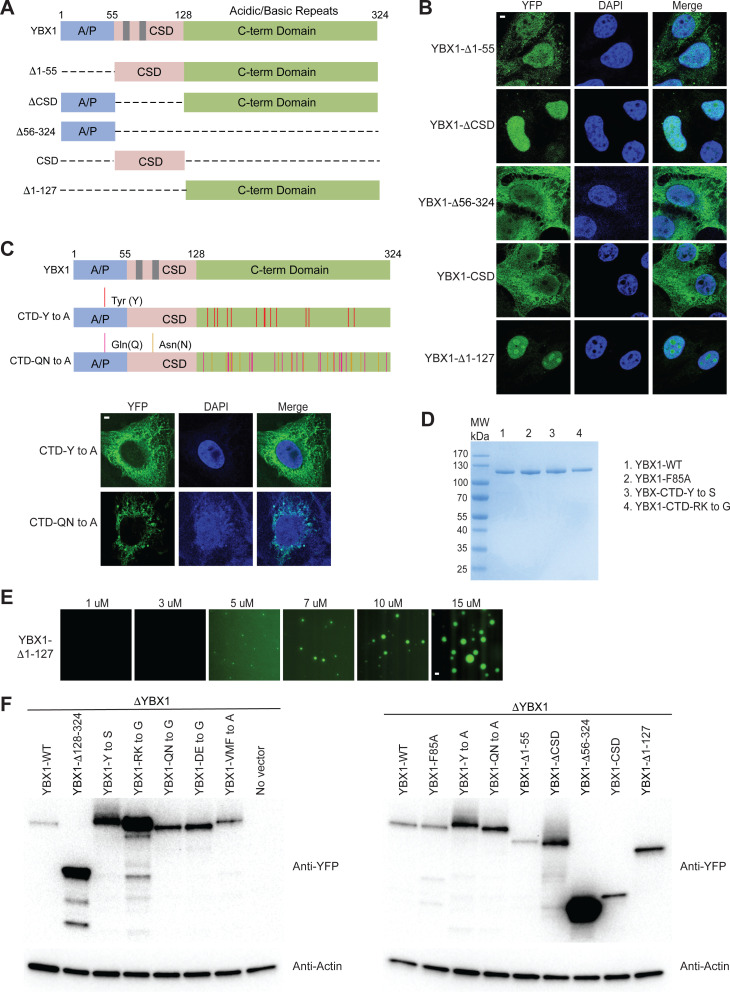
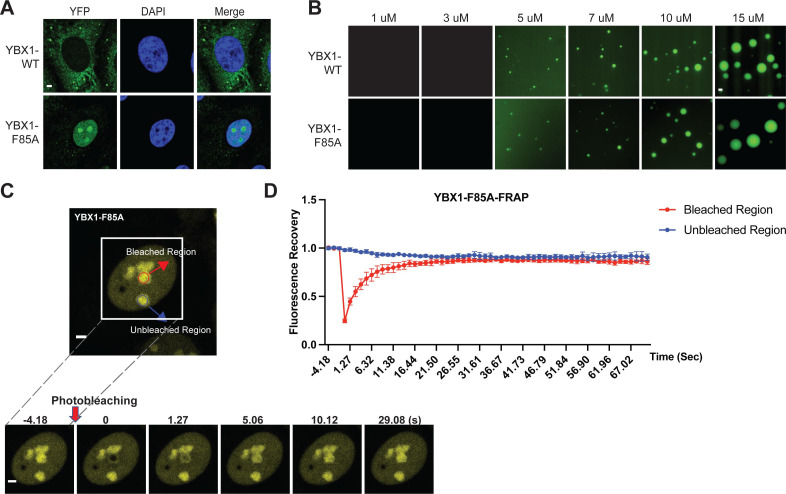
(A) Structural organization of YBX1. Top, IUPred, prediction of disordered protein regions; Middle, NCPR, net charge per residue with a sliding window of five residues; Net positive, blue, net negative, red; Bottom, visualization outputs for residue plots. (B) Schematic diagrams of different YBX1 mutants with the distribution of mutated amino acids. (C) Truncation mapping and identification of residues in YBX1 C-terminal IDR that are required for YBX1 condensation formation. YFP fused YBX1 wild type and mutants were introduced in ΔYBX1 U2OS cells by transient transfection and visualized by fluorescence microscopy. (D) Phase separation of YBX1 wild type and variants at the indicated concentrations. 6xHis-MBP-mGFP fused YBX1 wild type and variant proteins were purified from insect cells. Phase separation was induced by diluting the salt concentration from 500 mM to 150 mM in this assay. Scale bars, 3 µm.