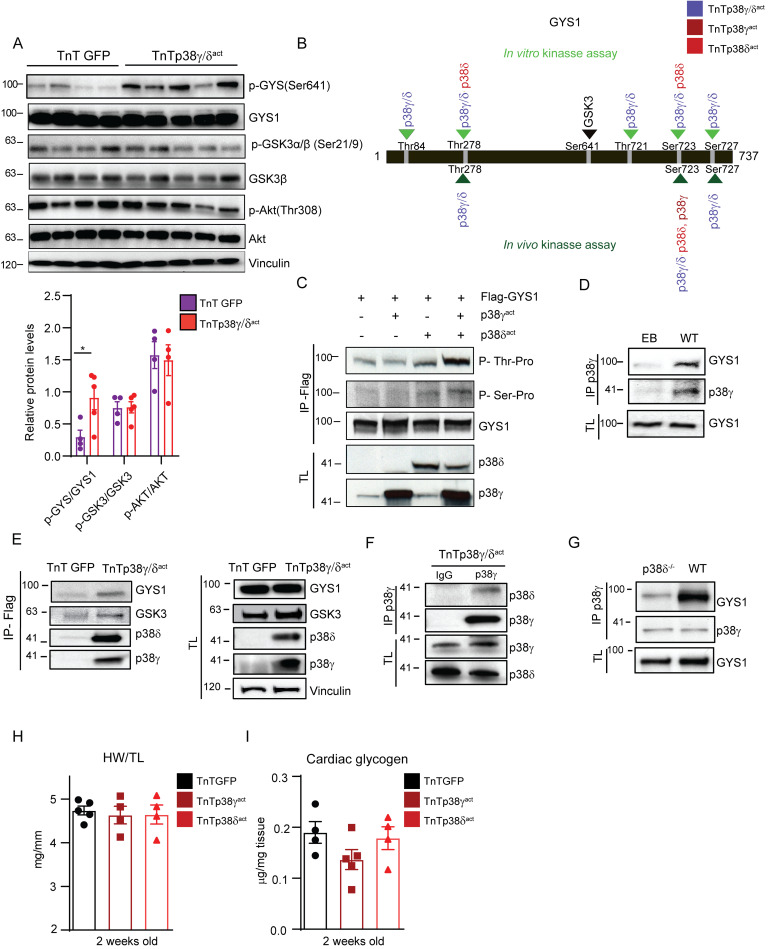
Fig 7. p38γ/δ cooperatively interact with GYS1 and GSK3 to promote GYS1 phosphorylation at its canonical site (Ser641).
(A) Immunoblot analysis of the Akt-GSK3–GYS axis in cardiac homogenates from AAV-cTnT-GFP-Luc (TnTGFP) or AAV-cTnT-p38γ/δact (TnTp38γ/δact) mice killed at PD14 with its respective quantification (lower panel). (B) Scheme showing GYS1 sites phosphorylated by p38γ, p38δ, or both, in an in vitro kinase assay (top, light green) or an in vivo kinase assay in HEK-293 cells (bottom, dark green). The GYS1 canonical site for GSK3 phosphorylation is Ser641 (shown in bold). Data are representative of at least 3 independent experiments (biological replicates). (C) In vivo phosphorylation of GYS1 in HEK-293 cells that had been transfected with Flag-GYS1 alone or together with p38γact or p38δ act, or both. Phosphorylation of transfected GYS1 was evaluated in the Flag-immunoprecipitate with phospho-MAPK substrates for Ser or Thr. TL before immunoprecipitation is shown as control. (D) Immunoprecipitation and immunoblot analysis of GYS1 and endogenous p38γ association in WT mice. (E) Immunoblot analysis of GYS1 and GSK3 in Flag-p38γ/δact immunoprecipitates from heart lysates, to detect interactions between these proteins and the exogenous p38γ/δact. (F) Immunoprecipitation and immunoblot analysis of the association between p38δ and p38γ in TnTp38γ/δact mice. (G) Immunoprecipitation/immunoblot analysis of the interactions between GYS1 and endogenous p38γ in WT or p38δ−/− mice. (H, I) Analysis of hearts from mice that were IV injected at PD1 with AAV-cTnT-GFP-Luc (TnTGFP), AAV-cTnT-p38γact (TnTp38γact), or AAV-cTnT-p38δact (TnTp38δact) and killed at PD14, showing (H) HWTL ratio and (I) cardiac glycogen content. Data are mean ± SEM (n = 10). One-way ANOVA coupled to Tukey posttest or Student t test. Raw data are given in S14 Fig. EB, empty bead; GSK3, glycogen synthase kinase-3; GYS1, glycogen synthase 1; HWTL, heart weight to tibia length; TL, total lysate; WT, wild-type.