Abstract
Sudden loss of smell and/or taste has been identified as an early symptom of SARS-CoV-2 2019 (COVID-19) infection, and presents an effective target for prompt self-isolation and reducing community spread. The current study sought to develop and test a novel, rapid, self-administered test to objectively measure smell and taste losses associated with COVID-19, and administered self-report questionnaires to characterise symptoms associated with COVID-19 in Singapore. Participants (N = 99) completed questionnaires to record recent changes in smell and taste ability. This was followed by the ‘Singapore Smell and Taste Test’ (SSTT), a personal, objective testing kit for daily self-assessment of smell and taste function at their place of residence. Seventy-two recruited participants were confirmed as COVID-19 positive at baseline, of which 58 completed the SSTT at home. Of these, 36.2% had objectively measured smell and/or taste loss. The SSTT measures of smell and taste function were positively associated with participants’ self-reported smell and taste acuity, and rated smell intensity of 6 common household items. This study presents the first application of the SSTT as a rapid, cost-effective, objective tool to self-monitor smell and taste function in a residential setting, and ensures comparability across individuals through the use of standardised stimuli. The SSTT has potential for future application in populations with limited access to formal COVID-19 testing as a self-administered objective method to monitor sudden changes in smell and taste, and to prompt early self-isolation, in order to reduce community transmission of COVID-19.
Abbreviations: COVID-19, Coronavirus disease 2019; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2; PCR, Polymerase Chain Reaction; SSTT, The Singapore Smell and Taste Test; SSTQ, The Singapore Smell and Taste Questionnaire; SNOT-22, 22-item Sino-Nasal Outcome test; VAS, Visual Analogue Scale
Keywords: SARS-CoV-2, COVID-19, Olfactory dysfunction, Gustatory dysfunction, Anosmia, Ageusia
1. Introduction
Sudden loss of smell (anosmia) and/or taste (ageusia) are now globally recognised as key symptoms of Severe Acute Respiratory Syndrome Coronavirus 2 infection (SARS-CoV-2) (causing the disease known as COVID-19) (American Academy of Otolaryngology-Head and Neck Surgery, 2020, ENT UK, 2020b, ENT UK., 2020a, European Rhinologic Society, 2020, Alert, 2020, Gane et al., 2020, Menni et al., 2020, Pellegrino et al., 2020), alongside fever, dry cough, fatigue and breathing difficulties (World Health Organisation, 2020). Many global public health bodies, including the Singapore Ministry of Health (2020), recommend that individuals at risk monitor their sense of smell and taste, and self-isolate and present for formal diagnostic testing should they experience any sudden changes (American Academy of Otolaryngology-Head and Neck Surgery, 2020, Centers for Disease Control and Prevention, 2020, ENT UK, 2020b, ENT UK., 2020a, Public Health England, 2020, World Health Organisation, 2020).
Data from self-report questionnaires collected across many countries and clinical populations demonstrate an association between sudden onset smell and/or taste loss and SARS-CoV-2 infection (Bagheri et al., 2020, Klopfenstein et al., 2020, Lechien, Chiesa-Estomba et al., 2020, Lechien, Chiesa-Estomba, Hans et al., 2020, Parma et al., 2020, Yan et al., 2020). Self-report questionnaire measures are often used because they are cost-effective and easy to implement at a large scale, but they can also be subjective, and prone to biases and inaccuracies. In particular, self-report measures of smell and taste often rely on individuals noticing these changes, which may result in under-reporting and not accurately reflect the prevalence of these symptoms. This is likely because the relationship between self-reported and objectively measured sensory function is typically low (Landis et al., 2003). A recent meta-analysis highlighted the need for objective sensory testing with standardised stimuli to identify and track COVID-19-related changes (Hannum et al., 2020).
This has been confirmed by a handful of studies that have objectively measured smell and taste changes using formal tests (Altin et al., 2020, Hintschich et al., 2020, Hornuss et al., 2020, Iravani et al., 2020, Lechien, Cabaraux et al., 2020, Lechien, Ducarme et al., 2020, Moein et al., 2020, Vaira, Deiana et al., 2020, Vaira, Hopkins et al., 2020, Vaira, Salzano et al., 2020, Villerabel et al., 2021). Recent meta-analyses report a considerable proportion of smell and taste disorders among individuals with COVID-19 (Agyeman et al., 2020, Rocke et al., 2020, Tong et al., 2020). Borsetto et al. (2020) reported an overall prevalence of 47% for changes in smell and taste, with 31% and 67% in severe and mild-to-moderate symptomatic patients respectively. As well as a high prevalence, particularly in milder cases (Aziz et al., 2020), smell and/or taste loss often occur early, sometimes presenting as the first symptom or the only symptom in otherwise asymptomatic individuals (Beltrán-Corbellini et al., 2020, Borsetto et al., 2020, Haehner et al., 2020, Hopkins et al., 2020, Iacobucci, 2020, Karimi-Galougahi et al., 2020, Kaye et al., 2020, Lee et al., 2020, Vukkadala et al., 2020). The onset of smell and/or taste loss is often sudden and, unlike other upper respiratory tract infections, frequently occurs in the absence of nasal obstruction (Gane et al., 2020, Gengler et al., 2020, Lechien, Chiesa-Estomba, Hans et al., 2020). Sudden smell and/or taste loss presents as a key symptom of COVID-19 that could prompt rapid self-isolation in otherwise asymptomatic individuals, who may risk unintentional spreading of the virus without exhibiting some of the more pronounced symptoms, such as dry cough or fever.
The available evidence on self-reported smell and taste loss with COVID-19 highlights wide variation in the prevalence of these symptoms, with values ranging from 3 to 98% for smell loss and from 6 to 63% for taste loss with SARS-CoV-2 infection depending on the population under study (Agyeman et al., 2020). Data from a retrospective review of medical records demonstrates lower prevalence numbers compared to the majority of self-report studies (Pellegrino et al., 2020). Furthermore, Hajikhani et al. (2020) reported that decreased sense of smell (hyposmia), and decreased (hypogeusia) or distorted (dysgeusia) sense of taste were more common than complete loss of smell (anosmia), and this gradation of symptoms may further explain the heterogeneity of recent findings. Although individuals may notice a sudden and complete loss of smell and/or taste, they may be less aware of subtler reductions in sensitivity over time. In this respect, objective measures of smell and taste sensitivity may be more accurate in identifying changes related to COVID-19, with pooled prevalence estimates of smell loss of 77% and 45% when measured through objective and subjective methods respectively (Hannum et al., 2020).
Recent findings from formal smell testing with the University of Pennsylvania Smell Identification Test (UPSIT) revealed that 98% of COVID-19 positive participants exhibited some smell dysfunction, with 25% presenting with anosmia and 73% with severe to mild olfactory dysfunction (Moein et al., 2020). The UPSIT and other traditional objective smell and taste tests are designed to provide a formal clinical diagnosis of sensory (dys)function. Hence, they are often time-consuming to administer and require delivery or instruction by a medical professional, making them impractical for widespread application of self-administered monitoring in a community setting. In addition, many of these approaches utilise test stimuli that are designed for repeated use, and become expensive when used only once in an effort to reduce cross-infection between subjects. Current social distancing requirements also restrict access to COVID-19 patients and require objective sensory tests to be self-administered with remote data collection. Although a formal clinical diagnosis with a Polymerase Chain Reaction (PCR) swab test is still necessary to confirm SARS-CoV-2 infection, early self-isolation is key to limiting the spread of the infection, and could be supported by a rapid, cost-effective, self-administered screening test with standardised stimuli for those who may not have immediate access to formal swab tests.
The current study sought to develop and test a novel, rapid, self-administered test, the Singapore Smell and Taste Test (SSTT), to objectively measure smell and taste losses associated with COVID-19. We aimed to create a brief testing procedure that could be easily administered in the home with minimal cost and participant burden. In the wake of the COVID-19 pandemic, and the pressing need to investigate smell and taste losses in COVID-19 populations, other home testing procedures utilising household items as items in a smell and taste test have been developed (Iravani et al., 2020, Vaira, Salzano et al., 2020). In the current situation, where traditional, expensive, clinically-administered smell and taste tests are impractical for use with restricted-access patients and in times of social distancing, these present a test than anyone can feasibly do, providing they have some of the specific household ingredients. However, we sought to further reduce the participant burden and time to complete our test by providing individuals with standardised stimuli in a testing kit. In addition, this reduces the risk of introducing unwanted stimuli variation or problems for comparability of smell and taste loss across individuals that can occur when participants are required to source and prepare their own test stimuli. With the SSTT, we present a method to assess smell/taste loss across individuals in a comparable way, with minimal participant burden and easy administration.
Thus, the current study presents the first application of the SSTT to objectively measure changes in smell and taste sensitivity, and compares responses to self-reported changes in sensory acuity using questionnaire approaches in the same participants.
2. Methods
2.1. Study design
In the current study, participants were asked to report on changes to their smell and taste sensitivity, and the onset of any changes, in the two previous weeks via a series of self-report questionnaires, and thereafter to prospectively track daily changes in smell and taste acuity using an objective self-assessment test. This self-assessment test objectively measured smell and taste acuity. The trial was registered at ClinicalTrials.gov (Ref: NCT04492904) and the study obtained ethical approval from the National Healthcare Group Domain Specific Review Board (DSRB) (Ref: 2020/00810). The study design, measures, and protocol are outlined fully in Sheen et al. (2020).
2.2. Recruitment procedure
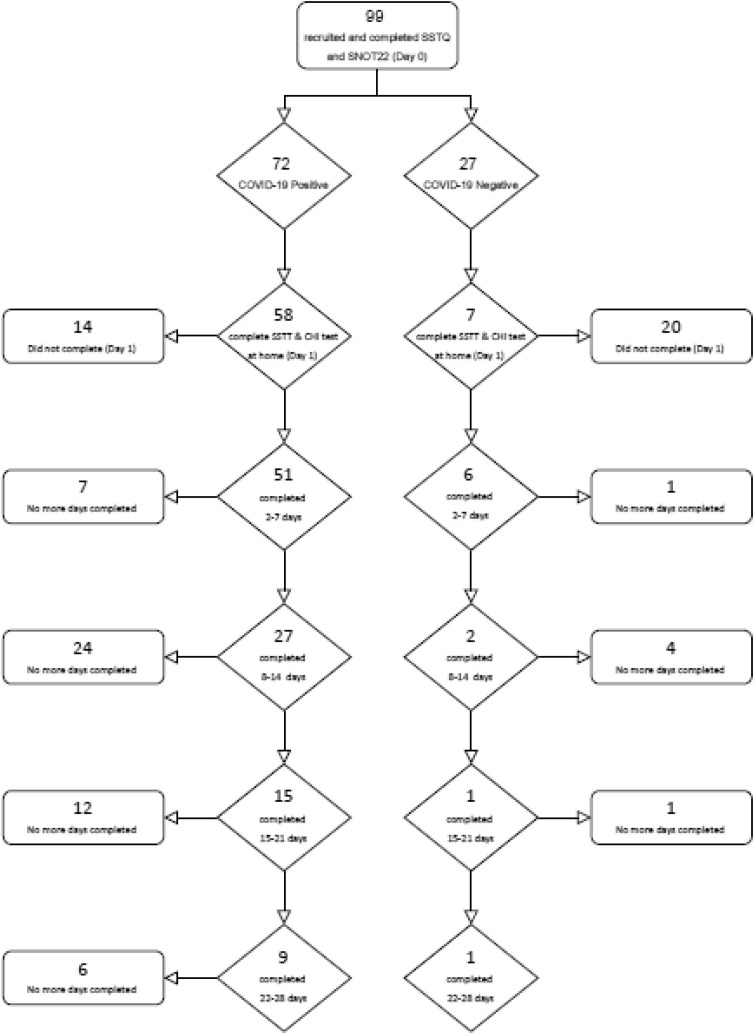
Participants were recruited from patients attending COVID-19 screening at the National University Hospital (NUH) and individuals from a Community Care Facility (CCF) who had been referred for a PCR test following suspected COVID-19, or who had tested COVID-19 positive on the day of recruitment into the study. As such, ‘Day 0′ represents the day that each participant underwent the PCR test and consented to participate. To confirm whether these individuals suspected of COVID-19 infection were positive, Nasal Swab PCR test results were confirmed for each participant via one-time access to their medical records. A small portion were identified as COVID-19 negative (see Fig. 1 ).
Fig. 1.
Flow chart of test completion by participants. displays the tests complete by each participant. Each day of testing included the SSTT and the Common Household Items Test. CHI = Common Household Item.
2.3. Measures and study procedure overview
On recruitment into the study (‘day 0′), participants completed the Singapore Smell and Taste Questionnaire (SSTQ) (Sheen et al., 2020) to document recent changes in smell and taste and the occurrence of other COVID-19 symptoms. The SSTQ was completed once at baseline and the full battery of questionnaires took approximately 20 min to complete. They also completed the 22-item Sino-Nasal Outcome test (SNOT-22) (Hopkins et al., 2009) to quantify the presence and severity of generalized nasal disorder symptoms. Participants were then given a demonstration of the Singapore Smell and Taste Test (SSTT), and completed this in the company of the experimenter (Sheen et al., 2020). Participants were then asked to complete this standardised self-administered smell and taste test procedure daily for up to 28 days, which included self-reported rating of their sense of smell and taste at that moment, and completing the SSTT. In total, the SSTT took less than 5 min to complete. Separately, participants selected one item from each of six categories of household items commonly found in Singapore from a pre-defined list and reported their perception of the smell (see Appendix for the full item list). This approach was based on a previously reported self-assessment measure of smell sensitivity using household items (Iravani et al., 2020). Due to a low compliance with the daily SSTT testing over the 28 days, there was insufficient complete data to report the longitudinal changes in smell and taste and these results are not discussed in the current manuscript.
2.4. Development of the SSTT
Participants each received an individual smell and taste test kit. The smell test involved two odour pens – a food odour (mango) and a non-food odour (detergent) – consisting of specifically-formulated commercially available odour mixtures chosen to avoid possible specific anosmia to individual odour compounds (Symrise AG). Odour-delivery pens that are widely used for odour delivery in commercial olfactory tests (Otto Hut, Germany) were chosen for home-use self-testing of olfactory function. The absorbent insert from each pen was dosed with a suprathreshold concentration of an odour mixture (food or non-food). Perceived odour quality and the intensity, consistency and stability of volatile bleed-rate was based on extensive pilot testing by both the flavour provider (Symrise AG) and at the Clinical Nutrition Research Centre to ensure a consistent odour stimulus for self-testing. For the self-administered taste test, participants were provided with four food-grade taste stimuli in powder form, with each selected to represent a standardised supra-threshold example of the four prototypical basic tastes: “sweet” (table sugar), “salty” (table salt), “bitter” (granulated coffee powder), and “sour” (lime powder).
The odour self-test applied similar test instructions to that used in the Yale School of Medicine “Jiffy” Test of Smell Sensitivity (Yale School of Medicine, 2020). Participants were asked to remove the pen lid and placed the odour pen 3 in. from their nose while breathing normally. Participants identify the smell from each pen and rate the perceived intensity on a visual analogue scale (VAS) from “not strong at all” to “extremely strong smell.” For the daily taste test, participants were asked to take a small amount of each item in a sequential monadic order on the tip of their tongue with a small spoon, and identify and rate the perceived intensity of the taste on a VAS from “not strong at all” to “extremely strong taste”.
2.5. Data analyses
Descriptive statistics were used to summarise the prevalence of COVID-19 and smell and taste loss (self-report and objectively measured) in the recruited sample. Composite smell and taste scores were calculated from VAS ratings of smell and taste from the SSTT. The average rated intensity from the two odours were calculated to produce a composite smell score, and the average rated intensity from the four basic tastants were calculated to produce the composite taste score. For the common household items smell test, the VAS ratings were summed across the six categories and averaged to produce a composite smell score. Participants had the aforementioned composite scores for each day they completed the SSTT. The cut-off criteria for smell and taste loss were informed by a previously published scoring system that used a similar approach, based on the Connecticut Chemosensory Clinical Research Centre (CCCRC) Test (Cain et al., 1983), in a COVID-19 population (Vaira, Salzano, et al. 2020). The scoring system is summarised in Table 1 , and we applied the same scoring system for both smell and taste. Responses on the household-item smell test included a mid-point of ‘normal’ (50), and participants scoring less than 45 were classified as having a reduced smell sensitivity.
Table 1.
SSTT scoring system.
| Composite Score | Smell Status | Taste Status |
|---|---|---|
| 90–100 | ‘Normal’ | ‘Normal’ |
| 70–89.9 | Mild hyposmia | Mild hypogeusia |
| 50–69.9 | Moderate hyposmia | Moderate hypogeusia |
| 20–49.9 | Severe hyposmia | Severe hypogeusia |
| 0–19.9 | Anosmia | Ageusia |
Linear regression analyses (with Bias-corrected and accelerated (BCa) 95% confidence intervals) were used to investigate whether objective composite scores were associated with self-reported smell and taste scores, and self-reported smell intensity of a selection of 6 household items. Composite scores from ‘day 1′ were used for these comparisons (i.e. the first day that participants conducted the SSTT independently in the residential environment). Participants were only included in these analyses if their first day of completing the SSTT in the home (‘day 1′) was no more than 7 days after the date of recruitment (‘day 0′). Results were significant at p < .05 and all analyses were conducted on IBM SPSS Statistics 27. All figures were created using GraphPad Prism 8.
3. Results
3.1. Participants
Due to strict restrictions on movement, low rates of community spread in Singapore, and strict restrictions on access to the vulnerable patient population, there was a lower rate of recruitment than expected. Furthermore, many participants did not complete the daily home test procedure for 28 days. We attribute this to a lack of direct in-person contact with participants and no follow-up requirements with the remotely administered protocol, the lack of financial incentives for participants to complete the full protocol remotely, and that many stopped completing the daily testing procedure once they had recovered from COVID-19, which usually occurs in the first 10–14 days post-infection. Table 2 presents the demographic information for the sample, and Fig. 1 display a flow chart showing test completion by participants. Ninety-nine participants were recruited (75 males, 24 females), with a mean age of 34.93(±11.61) years, of which 72 were confirmed COVID-19 positive and 27 were confirmed COVID-19 negative.
Table 2.
Participant demographics (N = 99).
| n | % | |
|---|---|---|
| Sex | ||
| Male | 75 | 75.8 |
| Female | 24 | 24.2 |
| Nationality | ||
| Indian / Bangladeshi | 61 | 61.6 |
| Singaporean | 30 | 30.3 |
| Filipino | 3 | 3.0 |
| Russian | 2 | 2.0 |
| Bruneian | 1 | 1.0 |
| Indonesian | 1 | 1.0 |
| Malaysian | 1 | 1.0 |
| Ethnicity | ||
| Bangladeshi | 47 | 47.5 |
| Chinese | 18 | 18.2 |
| Indian | 17 | 17.2 |
| Malaysian | 8 | 8.1 |
| Filipino | 4 | 4.0 |
| Russian | 2 | 2.0 |
| Bruneian | 1 | 1.0 |
| Indonesian | 1 | 1.0 |
| Pakistani | 1 | 1.0 |
| Education | ||
| No formal qualification | 3 | 3.0 |
| Secondary school | 30 | 30.3 |
| High school | 31 | 31.3 |
| Diploma/degree | 31 | 31.3 |
| Post-graduate qualification | 4 | 4.0 |
| Smoking status | ||
| Never smoked | 75 | 75.8 |
| Not currently a | 12 | 12.1 |
| Yes | 12 | 12.1 |
| Medical condition b | ||
| No | 89 | 89.9 |
| Yes | 10 | 10.1 |
| Medication c | ||
| No | 89 | 89.9 |
| Yes | 10 | 10.1 |
“Not currently” refers to the response “Not currently, but I have in the past”.
No medical conditions relating to chemosensory dysfunction or illness were reported. There were reports of high blood pressure (n = 2), asthma (n = 1), cardiovascular issues (n = 1), diabetes (n = 1), hypertension (n = 1), hypertension and diabetes (n = 1), hypothyroid and rheumatoid arthritis (n = 1), lupus (n = 1), and diabetes, hypertension, hypersensitivity lung disease, and benign prostatic hyperplasia (n = 1).
No medication relating to, or that would affect, chemosensory function was being taken by participants. Participants reported taking losartan (n = 1), metformin (n = 1), prednisone (n = 1), albuterol inhaler (n = 1), and one reported taking irbesartan, dutasteride, rosuvastatin and aspirin (n = 1), medication for hypertension (n = 1), anti-thyroid and rheumatoid arthritis (n = 1), hypertension and diabetes (n = 1), and one reported taking insulin as well as medication for hypertension (n = 1), and one participant’s response was unclear (wrote “less”) (n = 1).
3.2. Objective smell and taste loss with COVID-19 measured by the SSTT
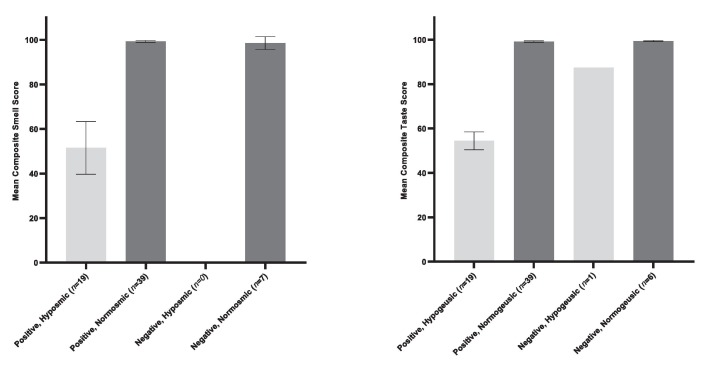
At ‘day 1′, 65 participants completed the SSTT, 58 of which were confirmed COVID-19 positive. The SSTT demonstrated that 22 participants exhibited smell and/or taste loss at ‘day 1′. Of those presenting with smell and/or taste loss, 21 were COVID-19 positive and 1 was COVID-19 negative (and had objectively measured taste loss only). Of the 21 COVID-19 positive patients, 17 exhibited both smell and taste loss, 2 exhibited smell loss only, and 2 exhibited taste loss only. Mean composite smell and taste scores split by COVID-19 test status and objectively measured smell and taste function at ‘day 1′ (‘normal’ or ‘hyposmia/hypogeusia’) are displayed in Fig. 2 .Fig. 3 .
Fig. 2.
Mean composite scores on the SSTT at ‘day 1′ by COVID-19 test outcome and objective smell/taste function at ‘day 1′ (‘normosmic/geusic’ or ‘hyposmic/geusic’) (N = 65). displays the mean composite smell and taste scores on the SSTT at ‘day 1′ split by COVID-19 test outcome (positive/negative) and objective smell/taste loss at ‘day 1′ (‘normosmic/norm-geusic’ or ‘hyposmia/hypogeusia’). One COVID-19 negative participant was identified as exhibiting moderate hypogeusia. Error bars are 95% confidence intervals.
Fig. 3.
Objective smell/taste function by category at ‘day 1′ for COVID-19 positive patients (N = 58) shows the number of COVID-19 positive participants in each category of smell and taste function as defined by our tiered scoring system.
3.3. Self-reported symptoms from the SSTQ and SNOT-22
At ‘day 0′, 60 COVID-19 positive participants reported suffering no symptoms currently or within the last 24 h on the SSTQ (83.3% of COVID positive participants, see Table 3 and Appendix). Furthermore, 62 COVID-19 positive individuals reported either no sino-nasal or related problems on the SNOT-22 (n = 6) or “Reduced Concentration” only (n = 56) (86.1% of COVID positive participants) (see Appendix). There was wide heterogeneity in the symptoms reported by participants, with the most common response being reduced concentration only.
Table 3.
Smell and taste symptoms self-reported on the SSTQ split by COVID-19 test outcome (N = 99).
| Current |
Last 24-hours |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Anosmia + Hypogeusia | Hyposmia only | No smell and taste loss | Anosmia + Hypogeusia | Hyposmia + Hypogeusia | Hyposmia only | Hypogeusia only | No smell and taste loss | ||
| Positive (n = 72) | No other symptoms | 1 | 2 | 60 | 1 | 0 | 2 | 0 | 63 |
| With other symptoms | 1 | 1 | 7 | 0 | 1 | 1 | 1 | 3 | |
| Negative (n = 27) | No other symptoms | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 15 |
| With other symptoms | 0 | 0 | 22 | 0 | 0 | 1 | 0 | 11 | |
Table 3 displays smell and taste symptoms self-reported on the SSTQ currently and within the last 24 h split by COVID-19 test outcome (positive/negative). aThese terms were rephrased in the SSTQ as follows: ‘Anosmia’ = ‘Smell loss (total), ‘Hyposmia’ = Smell loss (partial), ‘Hypogeusia’ = ‘Diminished taste’.
3.4. Comparing the SSTT to self-reported smell and taste sensitivity and the common household item smell test
Of the 21 individuals with objectively measured smell/taste loss at ‘day 1′ using the SSTT, only 16 self-reported a smell/taste loss score below 90. Composite scores on the objective smell test were positively associated with those on the self-report smell function questionnaires at day 1, B = 0.91 [0.73, 1.05], p =.001 (N = 65). Similarly, composite scores on the objective taste test were positively associated with those on the self-report taste function item, B = 0.73 [0.42, 0.94], p = .001 (N = 65).
There was less agreement with the common household items smell test. Of the 17 COVID-19 positive participants with smell and/or taste loss on the SSTT who completed the common household item test at ‘day 1′, only 2 participants reported reduced scores on the household items at baseline. The most common household items selected for smell assessment by our participants from each category were orange (fruit), hair shampoo (cleaning/cosmetic product), chicken rice (savoury food 1), onion (savoury food 2), garlic (spices), and toothpaste/menthol (trigeminal smells) (see Appendix). However, composite scores on the objective smell test was positively associated with self-reported smell intensity of the six common household items (composite score), B = 0.30 [0.09, 0.46], p = .008 (N = 58).
3.5. Adherence rates for completing daily smell and taste testing (SSTT)
On average, 7 of a possible 28 test days were completed by participants. All participants completed the daily smell and taste test battery at baseline (Day 0), but only 17 completed ≥ 14 days, and only 5 completed 27–28 days of testing. Of those with smell/taste loss at ‘day 1′, 17 completed more than one day of the SSTT at home (see Appendix).
4. Discussion
The current study presents the first application of the SSTT as a rapid, cost-effective, self-administered home-use test that could be used to track changes to smell and taste with COVID-19. These objective smell and taste measures identified losses in smell and taste sensitivity among COVID-19 positive participants, and aligned with self-report measures of smell and taste function. Our preliminary findings also provide initial insights into the lack of symptoms experienced by those with a mild SARS-CoV-2 infection who were otherwise healthy.
We found that smell and taste losses were typically exhibited together, as opposed to smell or taste loss alone. It may be that COVID-19 only impacts smell function, and the observed changes in taste sensitivity reflect smell-referred taste loss as opposed to impaired gustatory function (Hintschich et al., 2020). This has been suggested by authors investigating inflammation of, or damage to, olfactory regions with SARS-CoV-2 as a potential mechanism for the observed smell and/or taste losses with COVID-19 (Brann et al., 2020, De Melo et al., 2020, Hintschich et al., 2020, Meinhardt et al., 2021, Soler et al., 2020). Further research with larger cohorts will discern whether smell-referred taste loss or a mechanism of the infection itself is responsible for taste losses associated with COVID-19.
In the current study, we aimed to create a brief objective testing procedure that could easily be administered remotely with minimal cost as an initial tool to diagnose smell and taste losses associated with COVID-19. Our objective/standardized stimuli method shows advantages over many of the traditional self-report questionnaire methods. Previous investigations into smell/taste loss with COVID-19 in Singapore samples have relied on self-reported smell and taste function in clinical reports (Chua et al., 2020, Wee et al., 2020), which may be open to bias. In addition, several individuals were unaware of their smell and taste changes until they were tested, suggesting that these individuals would have been missed by any conventional self-report questionnaire. Despite this, we suggest it is still important to include self-report measures (such as the SSTQ) to capture concurrent symptomology associated with COVID-19 infection.
Other home testing procedures have been developed, including those that use common household items to create a smell and taste tests (Iravani et al., 2020, Vaira, Salzano et al., 2020). This approach enables remote, self-administered assessment of smell and taste, which is particularly valuable in times of social distancing and self-isolation, where in-person data collection is difficult. In the current study, the household item test was shown to be efficient, though it afforded slightly less control of the stimuli used for self-testing. In comparison, by utilising standardised stimuli, the SSTT does not require participants to source or prepare their own test stimuli. An advantage of this approach is that it reduces the risks of introducing unwanted stimuli variation or problems for comparability of smell and taste loss across individuals that can occur when participants are required to source and prepare their own test stimuli. The SSTT offers a method that can assess smell/taste loss across individuals in a comparable way, with standardised test stimuli and instructions that can be easily be administration remotely and cost-effectively.
Conventional clinical tests, such as Sniffin’ Stix (Wolfensberger et al., 2000), are effective at measuring changes in olfactory sensitivity and identification. However, these tests can be expensive to purchase and are not practical when administering a standardised test to a large population of COVID-19 infected participants, due to the high cost of each testing kit, and the inability to share or re-use test kits within a patient population due to the risk of contamination and the increased spread of the virus. Current evidence indicates that differences in smell/taste loss with COVID-19 are not at the perithreshold level, indicating that a formal clinical diagnostic threshold test may be unnecessary for the taste and smell self-testing required to prompt self-isolation. In this regard, the SSTT presents a cost-effective and objective approach to collect data remotely and consistently within a COVID-19 patient population.
In the early stages of self-diagnosis, the SSTT could be extremely useful in initiating prompt self-isolation and the seeking of a formal medical consultation, especially in lower-middle income countries where rapid, widespread access to formal PCR swab testing is not always available. The SSTT also has applications in monitoring smell and taste recovery in individuals suffering long-term effects of COVID-19 (commonly referred to as ‘long COVID’) (Callard and Perego, 2021, Klein et al., 2021, Mahase, 2020), and could assist in identifying individuals that need to seek further medical advice to manage chronic smell and taste losses or disorders. The stimuli of the SSTT have a shelf life of 1 year, so individuals could keep a testing kit in their household for use if they suspect sudden loss, or slow recovery, of sensory acuity. The SSTT could have application for the detection of smell and taste changes as part of the pandemic response, and more broadly to track sudden changes in sensory perception.
A limitation of the current study is that the response rates of participants completing the SSTT daily during the 4-week test period were low, with only a minority completing the all 28 days of testing. This led to challenges in interpreting the longitudinal changes in smell and taste over time from onset to full smell and taste recovery. Issues with compliance and low sample sizes have also been observed in other studies that have tracked smell and taste changes in COVID-19 patient populations (Vaira, Deiana et al., 2020, Vaira, Hopkins et al., 2020). In the current trial, this was most likely due to participants discontinuing daily testing after they had recovered from COVID-19, and regained their smell and taste. The current trial also offered no financial incentive for participants to complete the full protocol remotely on a daily basis, which may account for a lower motivation to continue with daily testing for the full 28-day period. Daily questionnaires were kept short to increase compliance, with the SSTT taking less than 5 min to complete. However, as part of the study, the Common Household Item Test was also completed daily, which may have been more effortful for participants to complete and extended the daily testing time. Another limitation is that we did not include a control group with another viral infection such as rhinovirus or influenza, both of which have anecdotally been associated with loss of smell from sinus congestion.
Our study is underpowered to draw conclusions on the national prevalence of smell and taste loss with COVID-19 in Singapore. Nevertheless, our preliminary findings suggest a prevalence rate that is slightly higher than those from previous clinical case reports in Singapore (∼22.7% for self-reported smell and taste loss (Chua et al., 2020, Wee et al., 2020)) and lower than studies elsewhere that have applied objective smell and taste testing procedures (Altin et al., 2020, Hintschich et al., 2020, Vaira, Deiana et al., 2020For instance, Gözen et al. (2021) found that with a sample of 59 COVID-19 positive participants, the rate of olfactory disorder as measured by the Sniffin’ Stix was 83%. Vaira, San Pietro, et al. (2020), using a household items testing kit (Vaira, Salzano, et al., 2020), found that of 138 COVID-19 positive patients, 84.8% had smell and taste loss within the first 4 days of infection. Mazzatenta et al. (2020) with in-hospital objective smell testing found that 95% of 100 COVID-19 patients exhibited smell dysfunction, with 47% exhibiting taste dysfunction.
Globally, prevalence rates of smell/taste losses reported to date have been diverse, ranging from 3 to 98% and 6–63% for smell and taste loss respectively depending on the population under study (Agyeman et al., 2020). This heterogeneity could reflect varying severities of infection at time of reporting within participant groups, the use of unstandardized test stimuli, different time-points in the course of SARS-CoV-2 infection at which measures of smell/taste were taken (e.g. during the recovery stage of infection), as well as the variabilities between study populations. Furthermore, smell and taste losses typically occur early in COVID-19 (Borsetto et al., 2020), with hyposmia, hypogeusia and dysgeusia often more common than complete loss (Hajikhani et al., 2020). Although individuals are likely to notice a sudden and complete loss of sensory function they may be less sensitive to subtler reductions, such that early symptoms could pass unnoticed and may not be captured by retrospective questionnaires.
Going forward, the SSTT could be applied to profile changes in smell and taste longitudinally and track onset, severity and recovery from COVID-19 smell and taste changes. However, the current data set showed poor follow up, particularly from those that had recovered from COVID-19 infection. Future research should consider approaches to incentivise patients to continue their daily self-assessment to better understand how viral infection affects smell, taste, and appetite over time. In addition, although we tested food and non-food odours, it could be interesting to investigate using a wider variety of odours in future, particularly in the context of long-COVID and persistent changes to smell and taste. Here, a scratch and sniff style self-test, such as the Brief Smell Identification Test (B-SIT) (Cao et al., 2021), may be a more appropriate approach when testing the persistence of olfactory changes over time in future.
In conclusion, the current study presents the first application of SSTT to objectively measure smell and taste function among COVID-19 positive participants. The SSTT, as a rapid, cost-effective test involving pre-made objective testing stimuli for ease of self-administration in a home setting, has widespread application as an early diagnostic tool for individuals who suspect sudden changes in their sensory acuity. Individual early diagnostic measures, such as the SSTT, will support national efforts in containing the spread of COVID-19 and reducing the emergence of clusters of infections as restrictions ease and population vaccination efforts are underway.
5. Ethics approval and consent to participate
The study (ref: 2020/00810) has been approved by the Domain Specific Review Board of the National Healthcare Group, Singapore.
6. Consent for publication
Not applicable.
7. Availability of data and materials
Study materials were published as supplementary materials in Sheen et al. (2020).
Funding
This work was supported by the Biomedical Research Council Singapore (BMRC) COVID-19 Research Fund, Development and implementation of Approaches for Tracking Chemosensory Changes as an early Marker of COVID-19 infection [grant number 12Al04lg1lA04] (awarded to C. G. Forde).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Symrise AG for providing advice on the home-use smell test stimuli and supplying the two odours used to test supra-threshold smell sensitivity in the COVOSMIA-19 trial.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.foodqual.2021.104482.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Agyeman A.A., Chin K.L., Landersdorfer C.B., Liew D., Ofori-Asenso R. Smell and taste dysfunction in patients with COVID-19: A systematic review and meta-analysis. Mayo Clinic Proceedings. 2020;95(8):1621–1631. doi: 10.1016/j.mayocp.2020.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altin F., Cingi C., Uzun T., Bal C. Olfactory and gustatory abnormalities in COVID-19 cases. European Archives of Oto-Rhino-Laryngology. 2020;277(10):2775–2781. doi: 10.1007/s00405-020-06155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Otolaryngology-Head and Neck Surgery. (2020). Anosmia, Hyposmia, and Dysgeusia Symptoms of Coronavirus Disease. https://www.entnet.org/content/aao-hns-anosmia-hyposmia-and-dysgeusia-symptoms-coronavirus-disease.
- Aziz M., Goyal H., Haghbin H., Lee-Smith W.M., Gajendran M., Perisetti A. The association of “Loss of Smell” to COVID-19: A systematic review and meta-nnalysis. The American Journal of the Medical Sciences. 2020 doi: 10.1016/j.amjms.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagheri S.H., Asghari A., Farhadi M., Shamshiri A.R., Kabir A., Kamrava S.K., Jalessi M., Mohebbi A., Alizadeh R., Honarmand A.A., Ghalehbaghi B., Salimi A., Firouzabadi F.D. Coincidence of COVID-19 epidemic and olfactory dysfunction outbreak in Iran. Medical Journal of the Islamic Republic of Iran. 2020;34(1)(62):1–7. doi: 10.34171/mjiri.34.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Corbellini Á., Chico-García J.L., Martínez-Poles J., Rodríguez-Jorge F., Natera-Villalba E., Gómez-Corral J., Gómez-López A., Monreal E., Parra-Díaz P., Cortés-Cuevas J.L., Galán J.C., Fragola-Arnau C., Porta-Etessam J., Masjuan J., Alonso-Cánovas A. Acute-onset smell and taste disorders in the context of COVID-19: A pilot multicentre polymerase chain reaction based case–control study. European Journal of Neurology. 2020;27(9):1738–1741. doi: 10.1111/ene.14273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borsetto D., Hopkins C., Philips V., Obholzer R., Tirelli G., Polesel J., Boscolo-Rizzo P. Self-reported alteration of sense of smell or taste in patients with COVID-19: A systematic review and meta-analysis on 3563 patients. Rhinology. 2020;58(5):1–7. doi: 10.4193/Rhin20.185. [DOI] [PubMed] [Google Scholar]
- Brann D., Tsukahara T., Weinreb C., Logan D.W., Datta S.R. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. Science Advances. 2020;6(31):eabc5801. doi: 10.11428/jhej1987.42.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain W.S., Gent J., Catalanotto F.A., Goodspeed R.B. Clinical evaluation of olfaction. American Journal of Otolaryngology-Head and Neck Medicine and Surgery. 1983;4(4):252–256. doi: 10.1016/S0196-0709(83)80068-4. [DOI] [PubMed] [Google Scholar]
- Callard F., Perego E. How and why patients made Long Covid. Social Science and Medicine. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao A.C., Nimmo Z.M., Mirza N., Cohen N.A., Brody R.M., Doty R.L. Objective screening for olfactory and gustatory dysfunction during the COVID-19 pandemic: A prospective study in healthcare workers using self-administered testing. World Journal of Otorhinolaryngology - Head and Neck Surgery. 2021 doi: 10.1016/j.wjorl.2021.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2020). Symptoms of Coronavirus. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html.
- Chua A.J.K., Chan E.C.Y., Loh J., Charn T.C. Acute olfactory loss is specific for Covid-19 at the Emergency Department. Annals of Emergency Medicine. 2020 doi: 10.1016/j.annemergmed.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Melo, G. D., Lazarini, F., Levallois, S., Hautefort, C., Michel, V., Larrous, F., Verillaud, B., Aparicio, C., Wagner, S., Gheusi, G., Kergoat, L., Kornobis, E., Cokelaer, T., Hervochon, R., Madec, Y., Roze, E., Salmon, D., Bourhy, H., Lecuit, M., & Lledo, P.-M. (2020). COVID-19-associated olfactory dysfunction reveals SARS-CoV-2 neuroinvasion and persistence in the olfactory system. BioRxiv, 2020.11.18.388819. http://biorxiv.org/content/early/2020/11/18/2020.11.18.388819.abstract.
- ENT UK. (2020a). Advice for patients with new-onset anosmia during COVID-19 pandemic. 20–22. https://www.entuk.org/sites/default/files/files/Advice for patients with new-onset anosmia during COVID-19 pandemic.pdf.
- ENT UK. (2020b). Loss of sense of smell as marker of COVID-19 infection. [DOI] [PMC free article] [PubMed]
- European Rhinologic Society. (2020). Information for Rhinologists on COVID-19. https://www.europeanrhinologicsociety.org/?page_id=2143.
- French Society of ENT (SFORL). (2020). Anosmia Alert COVID-19. https://www.sforl.org/wp-content/uploads/2020/03/Alerte-anosmie-COVID-19.pdf.
- Gane S.B., Kelly C., Hopkins C. Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology. 2020;58:1–4. doi: 10.4193/Rhino15.365. [DOI] [PubMed] [Google Scholar]
- Gengler I., Wang J.C., Speth M.M., Sedaghat A.R. Sinonasal pathophysiology of SARS-CoV -2 and COVID -19: A systematic review of the current evidence. Laryngoscope Investigative Otolaryngology. 2020;5(3):354–359. doi: 10.1002/lio2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gözen, E. ., Aliyeva, C., Tevetog, F., Lu, ˘, Karaali, R., Ilker, _, Balkan, I., Murat Yener, H., & Ahmet O ¨ Zdog ˘ An. (2021). Evaluation of Olfactory Function With Objective Tests in COVID-19-Positive Patients: A Cross-Sectional Study. https://doi.org/10.1177/0145561320975510. [DOI] [PMC free article] [PubMed]
- Haehner A., Draf J., Dräger S., De With K., Hummel T. Predictive Value of Sudden Olfactory Loss in the Diagnosis of COVID-19. Orl. 2020;82(4):175–180. doi: 10.1159/000509143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajikhani B., Calcagno T., Nasiri M.J., Jamshidi P., Dadashi M., Goudarzi M., Eshraghi A.A., Mirsaeidi M. Olfactory and gustatory dysfunction in COVID-19 patients: A meta-analysis study. Physiological Reports. 2020;8(18):1–7. doi: 10.14814/phy2.14578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannum M.E., Ramirez V.A., Lipson S.J., Herriman R.D., Toskala A.K., Lin C., Joseph P.V., Reed D.R. Objective sensory testing methods reveal a higher prevalence of olfactory loss in COVID-19–positive patients compared to subjective methods: A systematic review and meta-analysis. Chemical Senses. 2020;45(9):865–874. doi: 10.1093/chemse/bjaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintschich C.A., Wenzel J.J., Hummel T., Hankir M.K., Kühnel T., Vielsmeier V., Bohr C. Psychophysical tests reveal impaired olfaction but preserved gustation in COVID-19 patients. International Forum of Allergy and Rhinology. 2020;10(9):1105–1107. doi: 10.1002/alr.22655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C., Gillett S., Slack R., Lund V.J., Browne J.P. Psychometric validity of the 22-item Sinonasal Outcome Test. Clinical Otolaryngology. 2009;34(5):447–454. doi: 10.1111/j.1749-4486.2009.01995.x. [DOI] [PubMed] [Google Scholar]
- Hopkins C., Surda P., Kumar B.N. Presentation of new onset anosmia during the covid-19 pandemic. Rhinology. 2020;58(3):295–298. doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- Hornuss D., Lange B., Schröter N., Rieg S., Kern W.V., Wagner D. Anosmia in COVID-19 patients. Clinical Microbiology and Infection. 2020;26(10):1426–1427. doi: 10.1016/j.cmi.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacobucci G. Sixty seconds on anosmia. BMJ (Clinical Research Ed.) 2020;368 doi: 10.1136/bmj.m1202. [DOI] [PubMed] [Google Scholar]
- Iravani B., Arshamian A., Ravia A., Mishor E., Snitz K., Shushan S.…Lundström J.N. Relationship between odor intensity estimates and COVID-19 prevalence prediction in a swedish population. Chemical Senses. 2020;45(6):449–456. doi: 10.1093/chemse/bjaa034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Galougahi M., Raad N., Mikaniki N. Anosmia and the need for COVID-19 screening during the pandemic. Otolaryngology - Head and Neck Surgery (United States) 2020;163(1):96–97. doi: 10.1177/0194599820925056. [DOI] [PubMed] [Google Scholar]
- Kaye R., Chang C.W.D., Kazahaya K., Brereton J., Denneny J.C. COVID-19 Anosmia Reporting Tool: Initial Findings. Otolaryngology - Head and Neck Surgery (United States) 2020;163(1):132–134. doi: 10.1177/0194599820922992. [DOI] [PubMed] [Google Scholar]
- Klein H., Asseo K., Karni N., Benjamini Y., Nir-Paz R., Muszkat M., Israel S., Niv M.Y. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infections. A cohort study in Israeli patients. Clinical Microbiology and Infection. 2021 doi: 10.1016/j.cmi.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T., Kadiane-Oussou N.J., Toko L., Royer P.Y., Lepiller Q., Gendrin V., Zayet S. Features of anosmia in COVID-19. Medecine et Maladies Infectieuses. 2020;50(5):436–439. doi: 10.1016/j.medmal.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis B.N., Hummel T., Hugentobler M., Giger R., Lacroix J.S. Ratings of overall olfactory function. Chemical Senses. 2003;28(8):691–694. doi: 10.1093/chemse/bjg061. [DOI] [PubMed] [Google Scholar]
- Lechien J.R., Cabaraux P., Chiesa-Estomba C.M., Khalife M., Hans S., Calvo-Henriquez C., Martiny D., Journe F., Sowerby L., Saussez S. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head & Neck. 2020;42(7):1583–1590. doi: 10.1002/hed.26279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., De Siati D.R., Horoi M., Le Bon S.D., Rodriguez A.…Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): A multicenter European study. European Archives of Oto-Rhino-Laryngology. 2020;277(8):2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Chiesa-Estomba C.M., Hans S., Barillari M.R., Jouffe L., Saussez S. Loss of Smell and Taste in 2013 European Patients With Mild to Moderate COVID-19. Annals of Internal Medicine. 2020;173(8):672–675. doi: 10.7326/m20-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechien J.R., Ducarme M., Place S., Chiesa-Estomba C.M., Khalife M., De Riu G., Vaira L.A., de Terwangne C., Machayekhi S., Marchant A., Journe F., Saussez S. Objective olfactory findings in hospitalized severe COVID-19 patients. Pathogens. 2020;9(8):1–6. doi: 10.3390/pathogens9080627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Min P., Lee S., Kim S.W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. Journal of Korean Medical Science. 2020;35(18):1–6. doi: 10.3346/JKMS.2020.35.E174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahase E. The BMJ. BMJ Publishing Group; 2020. Covid-19: What do we know about “long covid”? 10.1136/bmj.m2815. [DOI] [PubMed] [Google Scholar]
- Mazzatenta A., Neri G., De Luca C., Marinari S., Porreca E., Cipollone F., Vecchiet J., Falcicchia C., Panichi V., Origlia N., Di Giulio C. Smell and taste in severe CoViD-19: Self-reported vs. testing. Testing Frontiers in Medicine. 2020;7 doi: 10.3389/fmed.2020.589409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinhardt J., Radke J., Dittmayer C., Franz J., Thomas C., Mothes R.…Heppner F.L. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nature Neuroscience. 2021;24(2):168–175. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- Menni C., Valdes A., Freydin M., Ganesh S., El-Sayed Moustafa J., Visconti A., Hysi P., Bowyer R., Mangino M., Falchi M., Wolf J., Steves C., Spector T. Loss of smell and taste in combination with other symptoms is a strong predictor of COVID-19 infection. MedRxiv. 2020 doi: 10.1101/2020.04.05.20048421. [DOI] [Google Scholar]
- Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: A biomarker for COVID-19. International Forum of Allergy and Rhinology. 2020;10(8):944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma V., Ohla K., Veldhuizen M.G., Niv M.Y., Kelly C.E., Bakke A.J.…Hayes J.E. More Than Smell-COVID-19 Is Associated With Severe Impairment of Smell, Taste, and Chemesthesis. Chemical Senses. 2020;45(7):609–622. doi: 10.1093/chemse/bjaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrino R., Cooper K.W., Di Pizio A., Joseph P.V., Bhutani S., Parma V. Coronaviruses and the Chemical Senses: Past, Present, and Future. Chemical Senses. 2020;45:415–422. doi: 10.1093/chemse/bjaa031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Public Health England. (2020). COVID-19: epidemiology, virology and clinical features - GOV.UK. https://www.gov.uk/government/publications/wuhan-novel-coronavirus-background-information/wuhan-novel-coronavirus-epidemiology-virology-and-clinical-features.
- Rocke J., Hopkins C., Philpott C., Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: A systematic review and meta-analysis. Clinical Otolaryngology. 2020;45(6):914–922. doi: 10.1111/coa.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen F., Tan V., Haldar S., Sengupta S., Allen D., Somani J., Chen H.Y., Tambyah P., Forde C.G. Evaluating the Onset, Severity, and Recovery of Changes to Smell and Taste Associated With COVID-19 Infection in a Singaporean Population (the COVOSMIA-19 Trial): Protocol for a Prospective Case-Control Study. JMIR Research Protocols. 2020;9(12) doi: 10.2196/24797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singapore Ministry of Health. (2020). UPDATES ON COVID-19 (CORONAVIRUS DISEASE 2019) LOCAL SITUATION. https://www.moh.gov.sg/covid-19.
- Soler Z.M., Patel Z.M., Turner J.H., Holbrook E.H. A primer on viral-associated olfactory loss in the era of COVID-19. International Forum of Allergy and Rhinology. 2020;10(7):814–820. doi: 10.1002/alr.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J.Y., Wong A., Zhu D., Fastenberg J.H., Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: A systematic review and meta-analysis. Otolaryngology - Head and Neck Surgery (United States) 2020;163(1):3–11. doi: 10.1177/0194599820926473. [DOI] [PubMed] [Google Scholar]
- Vaira L.A., Deiana G., Fois A.G., Pirina P., Madeddu G., De Vito A., Babudieri S., Petrocelli M., Serra A., Bussu F., Ligas E., Salzano G., De Riu G. Objective evaluation of anosmia and ageusia in COVID-19 patients: Single-center experience on 72 cases. Head and Neck. 2020;42(6):1252–1258. doi: 10.1002/hed.26204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Hopkins C., Salzano G., Petrocelli M., Melis A., Cucurullo M.…De Riu G. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head and Neck. 2020;42(7):1560–1569. doi: 10.1002/hed.26269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., Salzano G., Petrocelli M., Deiana G., Salzano F.A., De Riu G. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head and Neck. 2020;42(7):1–14. doi: 10.1002/hed.26228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaira L.A., San Pietro V., Vaira L.A., Hopkins C., Petrocelli M., Lechien J.R., Chiesa-Estomba C.M., Salzano G., Cucurullo M., Salzano F.A., Saussez S., Boscolo-Rizzo P., Biglioli F., De Riu G. Smell and taste recovery in coronavirus disease 2019 patients: A 60-day objective and prospective study. Journal of Laryngology and Otology. 2020;134:703–709. doi: 10.1017/S0022215120001826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villerabel C., Makinson A., Jaussent A., Picot M.-C., Nègre-Pagès L., Rouvière J.-A., Favier V., Crampette L., Morquin D., Reynes J., Le Moing V., Tuaillon E., Venail F. Diagnostic value of patient-reported and clinically tested olfactory dysfunction in a population screened for COVID-19. JAMA Otolaryngology-Head & Neck Surgery. 2021 doi: 10.1001/jamaoto.2020.5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vukkadala N., Qian Z.J., Holsinger F.C., Patel Z.M., Rosenthal E. COVID-19 and the otolaryngologist: Preliminary evidence-based review. The Laryngoscope. 2020;130(11):2537–2543. doi: 10.1002/lary.28672. [DOI] [PubMed] [Google Scholar]
- Wee L.E., Chan Y.F.Z., Teo N.W.Y., Cherng B.P.Z., Thien S.Y., Wong H.M., Wijaya L., Toh S.T., Tan T.T. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. European Archives of Oto-Rhino-Laryngology. 2020;277(8):2389–2390. doi: 10.1007/s00405-020-05999-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfensberger M., Schnieper I., Welge-Lüssen A. Sniffin’Sticks®: A new olfactory test battery. Acta Oto-Laryngologica. 2000;120(2):303–306. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- World Health Organisation. (2020). Coronavirus - Symptoms. https://www.who.int/health-topics/coronavirus#tab=tab_3.
- Yale School of Medicine. (2020). Yale School of Medicine Jiffy Test of Smell Sensitivity. https://yalesurvey.ca1.qualtrics.com/jfe/form/SV_3rzfStiKuEvtQvb.
- Yan C.H., Faraji F., Prajapati D.P., Boone C.E., DeConde A.S. Association of chemosensory dysfunction and COVID-19 in patients presenting with influenza-like symptoms. International Forum of Allergy and Rhinology. 2020;10(7):806–813. doi: 10.1002/alr.22579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Study materials were published as supplementary materials in Sheen et al. (2020).