Abstract
Image-guided robotics for biopsy and ablation aims to minimize procedure times, reduce needle manipulations, radiation, and complications, and enable treatment of larger and more complex tumors, while facilitating standardization for more uniform and improved outcomes. Robotic navigation of needles enables standardized and uniform procedures which enhance reproducibility via real-time precision feedback, while avoiding radiation exposure to the operator. Robots can be integrated with computed tomography (CT), cone beam CT, magnetic resonance imaging, and ultrasound and through various techniques, including stereotaxy, table-mounted, floor-mounted, and patient-mounted robots. The history, challenges, solutions, and questions facing the field of interventional radiology (IR) and interventional oncology are reviewed, to enable responsible clinical adoption and value definition via ergonomics, workflows, business models, and outcome data. IR-integrated robotics is ready for broader adoption. The robots are coming!
Keywords: biopsy, ablation, robotics, stereotactic navigation, treatment planning, interventional radiology
The role and impact of robotics in interventional radiology (IR) relies upon augmenting automation, standardization, and precision. To improve IR outcomes, we need to collectively arrive at better ways to deliver IR-related precision medicine so that it is both minimally invasive and accurate. Many devices, instruments, and software for needle or catheter navigation in IR might be considered related to “robotics” in some way. The term “robotics” means different things to different people. Some might conjure the terms navigation, fusion, stereotaxy, tracking, or automation. These facets of robotics all share the goal of using preprocedural imaging information to enable a more accurate or standardized needle-based procedure, such as percutaneous minimally invasive image-guided biopsy or ablation. Although robots improve IR physicians, they will not replace them.
Robotics is defined by the Robotic Institute of America as “a reprogrammable, multifunctional manipulator designed to move materials, parts, tools, or specialized devices through various programmed motions for the performance of a variety of tasks.” 1 Medical robots were first employed with stereotactic guidance in the 1980s with the CT-based stereotaxic brain biopsy 2 and also with a PUMA robot pre-programmed and customized to specific anatomy for urological surgery ( Fig. 1 ). 3 The most widely used robotic surgical system currently is the da Vinci robot (Intitutive), a “remote tele-surgery” device that consists of a robot with “end-effectors” (arms and hands) controlled and manipulated by a surgeon at a remote console ( Fig. 2 ). 2 3 This device offers a 3D view of the surgical field, 7 degrees of freedom (DOF), tremor stability, integrated fluorescence imaging, magnification, and has been applied across many specialties, including prostate resection, cholecystectomy, tumor resection, and hysterectomy. 2 3
Fig. 1.

Programmable robot (PUMA) adapted for medicine. (Reprinted with permission from Leal Ghezzi T, Campos Corleta O. 30 Years of robotic surgery. World J Surg 2016;40(10):2550–2557.)
Fig. 2.
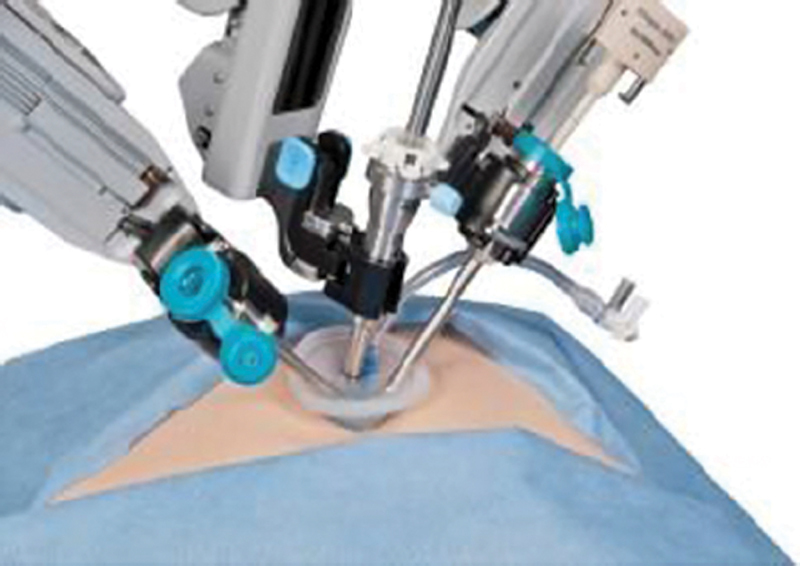
Da Vinci single-site surgical robotic system. (Reprinted with permission from Leal Ghezzi T, Campos Corleta O. 30 Years of robotic surgery. World J Surg 2016;40(10):2550–2557.)
Many medical robots share the navigational steps of planning, implementation, adjustment, and verification, and IR robots use imaging. The integration of imaging with robotics enables real-time intraprocedural feedback, which streamlines procedures, increases precision, and ultimately improves outcomes. 1 4 By standardizing procedures, robotics has the ability to minimize procedure times, allow less-experienced operators or interventional radiologists to perform complex procedures, reduce inter-operator human variabilities, and increase accuracy. 1 4 For needle-based IR procedures, such as biopsy and ablation, accuracy enhances capabilities and improves outcomes, ideally via fewer needle manipulations or complications, less radiation, and faster procedures able to deliver correct tissue biopsy or a complete ablation margin. When integrated into a treatment plan, accuracy may enable successful composite multiprobe ablation of larger and more complex tumors or “planned treatment volumes.” 1 4
A shortage of skilled operators during a surge of clinical need might also drive the adoption of IR robotics. Such could be the case for the personalization of biopsy, for which treatment decisions depend on customized patient-specific and tumor-specific accurate biopsy to better characterize a specific target beneath a sea of biomarker heterogeneity within tumors. IR operators also vary in experience, skillsets, hand–eye spatial awareness, and reproducibility and are subject to human factors of decision-making, estimation of needle angles, and cognitive multimodality registration or “guess/estimation.”
Cost-Effectiveness
IR robotics must prove added clinical value, cost-effectiveness, ease of use, ergonomics, and successful standardization to be widely applied. In general, outcomes research in medical robotics has lagged behind clinical adoption. Adoption of any novel technology in IR will be broader and more welcome when that new device has undergone practical utility or cost-effectiveness studies that define value or critically examine benefit in economic terms using standard cost outcomes metrics. Outcomes and cost models may be derived from repeated use of robotics for a specific procedure being performed in a very specific population (such as liver lesions in the 3–7 cm range). Other metrics applicable to IR robotics to better define cost-effectiveness might include cost–benefit ratio, cost utility, overhead costs, hospital costs, departmental costs, administrative costs, quality-adjusted life years, cost-effectiveness ratios, cost per patient, cost per diagnosis, time per procedure, and radiation dose per procedure. Models for examining cost savings—such as from fewer complications, faster procedures, more standardized procedures, or more reimbursement—can be challenging and full of broad assumptions. This is especially true when applied across a diagnosis, practice, department, hospital fiscal plan, or healthcare system. Investments required to commercialize IR robotics often require a sturdy and defendable business plan which must justify the added costs of hardware and/or disposables. Implementing new technology can also introduce personnel and workflow disruptions, learning curves, or ergonomic challenges as well. Although new technologies are commonly introduced to IR practice, a more thorough understanding of the downstream cost impact may better fulfill the promise of IR, enabled by judicious use of precision image guidance and minimal invasion. To be broadly adopted, robotics for IR must prove more effective with ultimately reduced overall costs to the healthcare system. The horse must lead the cart—not vice versa.
IR robotics should also not reproduce the experience of surgical robotics. Driven largely by patient and physician demand and local competition, surgical robots were innovated and widely adopted despite their equivocal marginal benefit, minimal comparative effectiveness research, and lack of convincing scientific evidence (some of which came later on). 5 Thus, rapid adoption of high technology surgical robotics occurred without evidence of superiority, even when higher costs were demonstrated. 6 IR robotic innovators can, and must, do better. Unlike a surgeon, an IR operator is always working below the skin, trying to make use of (and better apply) imaging guidance. Imaging guidance should be improved by automation, standardization, and precision, but it needs to be proven rather than assumed.
Uses within IR: Biopsy and Ablation
Biopsy
Robotics can be used to increase the precision and accuracy of image-guided ablation and biopsy, hopefully improving outcomes. Image-guided biopsy visualizes tissues and allows physicians to obtain samples of histological tests through a minimally invasive procedure relying upon image guidance and navigation. 7 However, accurate needle placement is crucial for accurate diagnosis, especially when critical structures (lung, bowel, nerves, vessels) are in proximity to the target. The success of the biopsy is dependent on the accuracy and reproducibility of placement, which is, in turn, tied to the operator's experience, hand–eye coordination, and spatial awareness. 7 Accurate visualization and needle placement in complex areas often requires intraprocedural scans and needle repositioning, exposing the patient and physician to radiation, especially with real-time computed tomography fluoroscopy (CT-fluoroscopy) or cone beam CT (CBCT) guidance. 7 With image-guided remote joystick robotics, the robot end-effector (e.g., needle holder or “hand”) is in the X-ray beam (instead of the operator's hands). One must also consider scatter from the components of the device that are in the beam, however.
Ablation
Tumor ablation in the liver, kidney, bone, and lungs is—to various degrees—both minimally invasive and image guided. 8 Image guidance is often only as effective as the operator's ability to imagine where the needle should be, and then use suboptimal and limited two-dimensional feedback to attempt to accurately place the needle at that specific point in space, which may not always be possible with high accuracy or precision. Ablation may have increased roles in future oncology treatment regimens, as it activates the immune system, increases permeability to enhance drug delivery, and can be combined with other therapies. 8 9 Microwave ablation (MWA) has been used more commonly in recent years for liver and lung ablation, in part due to being faster than radiofrequency ablation (RFA) and cryoablation, with greater energy output and larger treatment volumes per needle, making it attractive for larger tumors that require composite burns or tumors near blood vessels that might be susceptible to convective heat sink. 8 Such technologies for composite treatments are all dependent on multiple needle placements with simultaneous treatment or repositioning, all of which may be improved with semiautomated robotics. 10
Ablation is most effective when treating fewer smaller lesions typically less than 3 to 5 cm in diameter, in areas of less complex anatomy. This technique becomes less effective in large or multicentric lesions and locations of poor visibility. The accuracy of the ablation needle placement translates to covering a wide enough volume of ablation to avoid recurrence of the tumor. Accuracy also avoids critical structures and prevents complications, such as bleeding and perforation. 7 11 12 13 Incomplete treatment may also anger cancer cells, resulting in a pro-tumorigenic immune and inflammatory milieu. 14 Although partly speculative, accuracy may help “attain a clean margin,” while tipping the balance toward tumoricidal immunomodulation. Imaging is used to identify the planned treatment volume (target tissue), insert and guide the needle, and localize surrounding structures. Robotics could play a role in enabling the standardized and reproducible use of precision imaging information, by closing the human gap between virtual planning and actual needle delivery.
Imaging Platforms
There are various imaging platforms that can be integrated with robotics. CT and magnetic resonance imaging (MRI) provide high-resolution three-dimensional imaging for biopsy and ablation. 1 Although CT guidance is more established than MR guidance, it still has some disadvantages, such as radiation exposure, ergonomic constraints of the gantry, and needle repositioning that increase procedure time and radiation exposure. MR-guided procedures offer the additional advantages of real-time feedback with parameters such as blood flow, diffusion imaging, and tissue temperature and oxygenation. 1 MR robots must, however, be designed with pneumatic drivers or MR-compatible components, as well as MR-compatible equipment, which may increase cost and complexity. Ultrasound (US) provides real-time feedback for guidance without ionizing radiation, but US robots have not been as widely developed as CT, MR, or CBCT-guided robots. Specifically, transrectal US has been used in combination with surgical robots, such as the Da Vinci, but US robots have not been widely applied in IR. 1 It is possible that low-field MRI will drive down costs and promote special purpose custom application interventional MRI robots. Fringe field MRIs have also recently been FDA-cleared for office-based prostate interventions (e.g., Promaxo), where the low fringe field is used for registration (and to obviate fusion biopsy requiring rectal ultrasound and EM tracking).
Goals of IR Robotics
Given the importance of accurate needle insertion and guidance for both ablation and biopsy, the implementation of robotics is an attractive step for IR. The use of robots to navigate and guide needles may lead to more standardized and uniform procedures. This should, in turn, improve treatment outcomes, broaden indications, and decrease the learning curve by making procedures more standardized, reproducible, and reliable. Robotics allows for the needle to be guided remotely and provides more accurate navigation with greater dexterity than manual needle repositioning (especially for patients with complex anatomy). Once past the learning curve, efficiency improves by decreasing the number of scans needed. 1 Also, robotic-controlled or joystick-remote imaging allows the physician to track the placement of the needle and guide the procedure with real-time feedback, while avoiding radiation exposure. 1 15 16
The most impactful benefit of IR robotics, however, may be standardization, whereby robots make us better at what we do, equalize operators, and render procedures reproducible. Procedure outcomes may become more closely associated with treatment rather than with institution-specific or operator-specific techniques. Even novice trainees may ablate tumors like experts, with short learning curves through the use of stereotactic navigation. 17 In addition, the use of automated precision devices for ablation may result in successful complete tumor cell death in almost all liver cancer nodules, even those greater than 3 cm. 18
Techniques
IR robots need more than just hardware. They also rely upon integrated software, registration tools, DOF, needle pathway plans, multimodality integration, and ergonomic workflows ( Table 1 ).
Table 1. Steps for automated or robotic treatment planning.
| Pre-plan path, choose modality, choose tool, # needles |
| Same-day plan |
| Segment |
| Register |
| Predict |
| Navigate and guide needles |
| Adjust, adapt, and learn iteratively |
| Monitor |
| Confirm and verify |
Registration is the process of matching points (in images or in physical space), to reference the physical working space to the procedural imaging space and to the preprocedural images of choice that outline critical anatomy or tumor margins. Procedural devices (needles or ultrasound transducers) may also be registered. Image registration involves image-to-localizer registration and image-to-patient registration. Image-to-localizer registration allows the localizer to share the same coordinate system with multiple image modalities, especially in image fusions such as MRI-TRUS fusion. 19 If the localizer is visible on the image, the registration may be automatic. Image-to-image registration may integrate preoperative image data with intraoperative image data, 20 playing an important role in image navigation by linking imaging with the patient's body via a shared XYZ coordinate system. 21 In general, there are two different methods of image registration: frame or frameless registration. 22 In frame or stereotactic registration, each CT-visible fiducial marker is in a predefined position in relation to the frame (with known location). Image registration is thus enabled via the known geometry of the fiducial markers. 23 These unique 3D markers may be integrated within the robotic end-effector as well, and registration occurs when the end effector is imaged (e.g., with CT).
While imaging modalities can provide preoperative anatomical information for procedural planning, challenges remain, as the physician often needs more planning information to decide the location of the skin-entry points, as well as the angle and depth of successive insertions. The physician also needs real-time position and orientation information of the interventional devices in relation to moving or breathing organs. Surgical tracking devices, localizers, or respiratory gating are used to increase accuracy and safety, but the challenges of motion and deformation remain incompletely solved.
Common tracking systems are optical, electromagnetic, or mechanical. Optical tracking systems require direct “line-of-sight” throughout an intervention and were quickly adopted because of their high accuracy and large field of view. 24 Electromagnetic tracking systems use electromagnetic fields to localize electromagnetic sensors without requiring direct line-of-sight. 25 However, nearby metal may distort the magnetic fields critical to this system. 26 Mechanical tracking is a method that avoids such risk of image distortion. 27 Encoded joints are mechanically connected to a rigid structure such as the table (iSYS), the floor (Perfint Healthcare), the CT gantry frame (Pinpoint), or the patient (XACT Robotics). All of these have their pros and cons. Image registration in robots should consider the DOF, which relates to how many spatial and rotational aspects are tracked (six DOFs—three planes of translation along XYZ axes and three planes of rotation around XYZ axes).
Early IR Robots
Why don't we all have robots in CT or CBCT IR right now? It may be helpful to look back at the history of robotics, to better understand why some died slow deaths in academia or the marketplace, and why IR robots may be different. Hurdles for many robots included regulatory concerns with redundant safety measures, especially when using an industry-type of robot (designed for speed and force) in a healthcare setting, where speed and force might have perceived safety weaknesses. Business models may not fit a disposable model or be justified as an add-on to an existing CT or CBCT, without hard value outcomes data.
In 1985, an early PUMA robot was used for a neuro-biopsy. Aesop, Zeus, RoboDoc, and finally da Vinci all paved surgical robotics pathways. IR robots date back at least two decades. 3 28 IR robots were conceived to be predictive, precise, patient-specific, planned, and enabled by fusion or stereotaxy. Some of the earliest robotic systems used in IR for percutaneous interventions were designed to guide and reposition the needle and include the following: AcuBot PAKY-RCM, Innomotion, Mitsubishi RV-E2, Kawasaki, DLR/KUKA LWR, and Image Guide. 28
The AcuBot PAKY-RCM was developed approaching the year 2000 and had 6 DOF, 2 mm precision, was controlled remotely, and was used with CT or fluoroscopic guidance. It contained a radiolucent needle driver, remote center of motion (“RCM”) module that oriented the needle, an XYZ Cartesian registration stage to position the needle tip, and a table-mounted passive arm. 28 General electric had a spinoff (image guide) that mounted the robot on the CT gantry.
The Innomotion CT/Innomedic MRI contained a 6-DOF arm ( Fig. 3 ). Unlike the AcuBot or iSYS1, however, the Innomotion had a large control unit and could not easily be used to select different entry points. 28
Fig. 3.
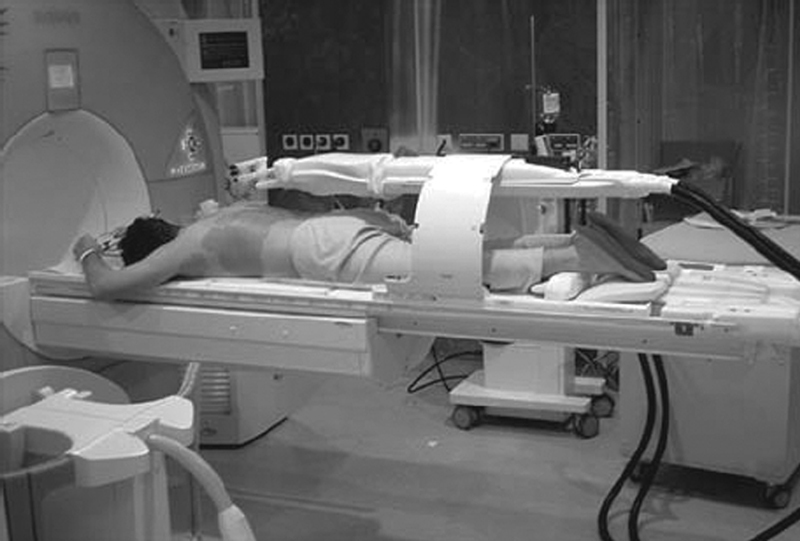
Innomotion MRI robotic system. (Reprinted with permission from Kettenbach J, Kronreif G. Robotic systems for percutaneous needle-guided interventions. Minim Invasive Ther Allied Technol 2015;24(1):45–53.)
The Mitsubishi RV-E2 and the Kawasaki were 6-DOF robots that used a CT-compatible end-effector or custom ablation end effector. Although these robots had high accuracy in phantom experiments, regulatory obstacles complicated clinical use. 28
DLR/KUKA LWR contained an optical navigation system and robotic arm on a mobile platform ( Fig. 4 ). However, the optical navigation camera's acquisition speed slowed down registration through reference frame grabbing/tracking. 28
Fig. 4.
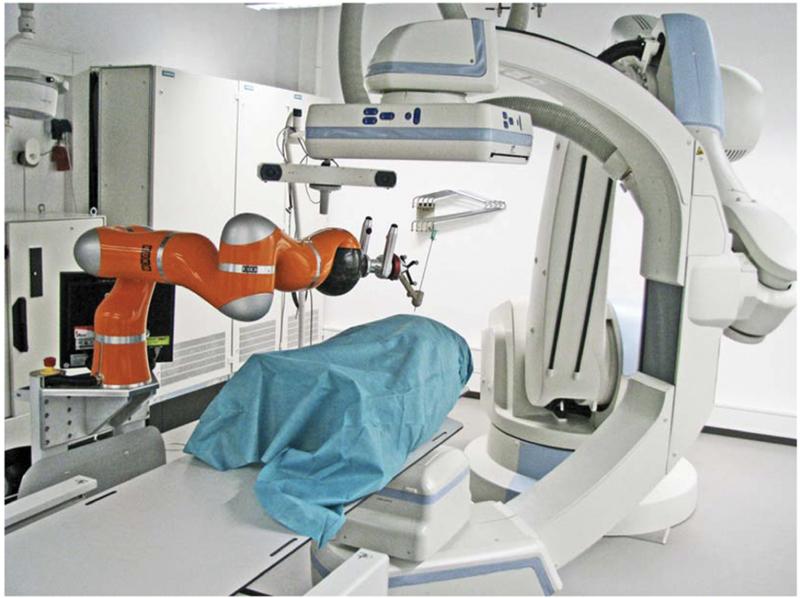
Robot (KUKA robot) with CBCT fluoroscopy system (Artis Zeego, Siemens Healthcare, Erlangen, Germany). The CBCT also has internal robotics to help position the C-arm. (Reprinted with Creative Commons Open Access from Tovar-Arriaga S, Vargas JE, Ramos JM, Aceves MA, Gorrostieta E, Kalender WA. A fully sensorized cooperative robotic system for surgical interventions. Sensors 2012;12(7):9423–9447.)
Current Applications
Current IR robot development efforts retain the goals of standardizing or improving needle procedures, via varying degrees of image integration (needle holder vs. needle driver, real-time fluoro-adjustment vs. active needle steering, simple placement vs. composite stereotactic ablation) with various mounting and registration schemes.
Stereotactic Techniques
Stereotactic navigation provides automatic robotic needle guidance and is amenable to multiple needle placements required for complex composite ablation of larger tumors. One such system for CT-guided ablation, CAS-ONE (CAScination AG; Fig. 5 ), has 6 DOF, needle detection, error calculation between the needle and the target zone, 9 29 30 and post-ablation completion/margin detection software.
Fig. 5.
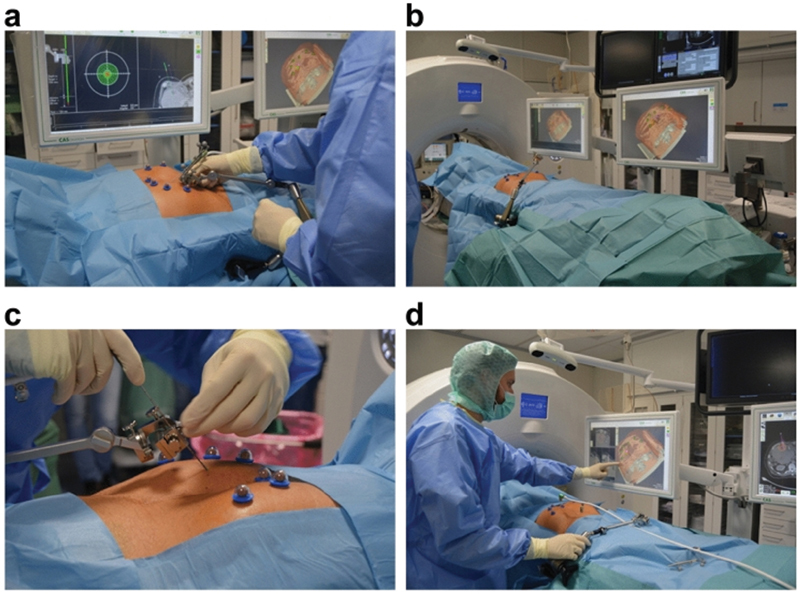
CAS-ONE (CAScination AG) robotic system. ( a ) Setup of aiming device; ( b ) positioning of device and patient; ( c ) probe setup; ( d ) monitor is active and pad/touch screen is covered in sterile material. (Reprinted with Creative Commons Open Access from Schaible J, Lürken L, Wiggermann P, et al. Primary efficacy of percutaneous microwave ablation of malignant liver tumors: comparison of stereotactic and conventional manual guidance. Sci Rep 2020;10(1):18835.)
The pre- and post-ablation images can also be overlaid to provide a 3D picture of the coverage of the tumor by ablation and to prompt re-ablation if necessary. Using this robotic system yields a higher primary efficacy rate—the percentage of a tumor destroyed after one ablation session—compared with manual guidance 30 and has a repositioning rate of just 1%. 9 This precise technique allows for better visualization of the ablation zone and safer access to lesions in areas of complex anatomy. Moreover, the tumor can be visualized in 3D and needles can be readjusted as needed. 9 29 30 Reto Bale in Innsbruck has also pioneered a variation of stereotaxy via the use of the Stealth surgical navigation station (Medtronic) adapted with optical tracking of rigidly mounted needle guides for precision placement, even by trainee operators, providing evidence that standardization is possible with such systems.
Table Mounted
The iSYS and the PAKY-RCM are mounted to the CT table and can orient a needle from the skin entry point while the needle driver advances the needle (controlled manually by the physician or through integration between computer and CT image). The robotic system uses CT fluoroscopy or CBCT to select a target and insert and advance the needle. Extra preparation time may be required compared with manual CT guidance due to the time required to attach the robot to the CT table, especially with early experience. 16
One table-mounted system (needle positioning system = NPS; DEMCON Advanced Mechatronics) was tested in a randomized controlled trial versus freehand for MWA of liver tumors. This system contains a robotic arm that moves on a rail that is parallel to the CT table (in a fixed configuration). No repositioning was necessary with the robotic system, whereas up to seven repositionings were required (per procedure) when inserting the needle manually. The robot also increased accuracy for out-of-plane targets. The targeting time was slightly longer than for manual guidance. 12 Such randomized controlled trials are to be recommended for the high level of data evidence produced.
Floor Mounted
The MAXIO (Perfint Healthcare) is mounted to a floor plate near the CT table for stable registration. The physician selects the probe specifications and target, and using a preprocedural CT, the software provides an entry point, trajectory, segmented 3D image, and ablation zone that the physician can manually adjust ( Fig. 6 ). The robotic arm then follows the plan and moves from the docked position to the entry point. 7 In tests, using this system with RFA provided greater coverage of the target area and reduced variability of accuracy compared with manual CT-guidance. Specifically, robotic systems show better targeting when the entry point and target are oblique or out of plane. 7 In fact, for MWA, the primary efficacy rate was 88% compared with 76% for freehand. 10 Some limitations of this setup include difficulty accessing one side of the CT table when the platform is set up, reduced tactile function of the needle because of the tightness of needle holder clamp guides, and limited access to craniocaudal targets. 7
Fig. 6.
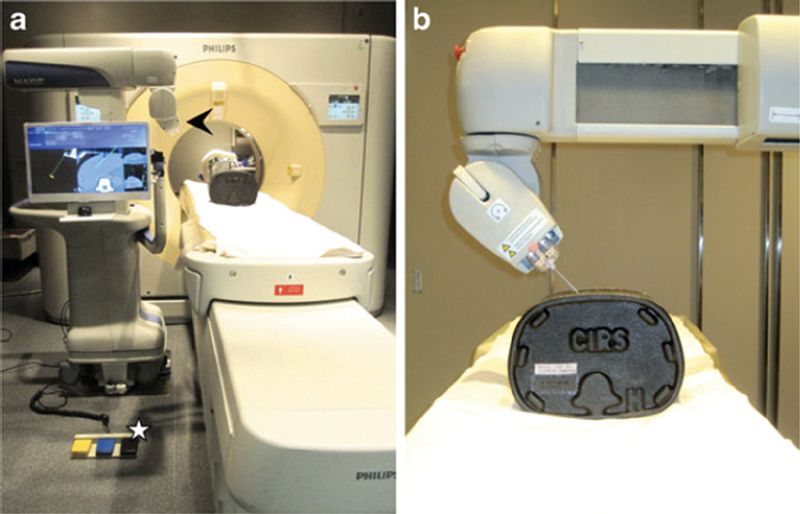
MAXIO (Perfint Healthcare) robotic system. ( a ) Robotic system is stabilized with a floor plate next to CT table; ( b ) robotic arm and hand (end effector) grip and guide needle. (Reprinted with permission from Koethe Y, Xu S, Velusamy G, Wood BJ, Venkatesan AM. Accuracy and efficacy of percutaneous biopsy and ablation using robotic assistance under computed tomography guidance: a phantom study. Eur Radiol 2014;24(3):723–730.)
The Quantum Surgical SAS (Montpellier, France) robotic system was reported in preclinical work for needle insertion assistance ( Fig. 7 ). 31 This robotic system consists of a mobile robotic cart with a robotic arm, a display cart with a touchscreen, a navigation cart with an optical tracking camera, a needle guide, and a patient reference that monitors the motion of the patient. 31 This model showed high accuracy, which was the same for both novice and experienced operators. 31
Fig. 7.

Quantum Surgical SAS (Montpellier, France) robotic system. (Reprinted with Creative Commons Open Access from Guiu B, De Baère T, Noel G, Ronot M. Feasibility, safety and accuracy of a CT-guided robotic assistance for percutaneous needle placement in a swine liver model. Sci Rep. 2021;11(1):5218.)
The Zerobot (Medicalnet Okayama) is also a floor-mounted CT robot with high accuracy and minimal radiation exposure ( Fig. 8 ). 32 The Zerobot can be used for out-of-plane insertions with high accuracy in phantom and animal experiments compared with manual insertion. 33
Fig. 8.

Zerobot robotic system. (Reprinted with Creative Commons Open Access from Komaki T, Hiraki T, Kamegawa T, et al. Robotic CT-guided out-of-plane needle insertion: comparison of angle accuracy with manual insertion in phantom and measurement of distance accuracy in animals. Eur Radiol 2020;30(3):1342–1349.)
Patient Mounted
The XACT Robot is miniature, hands-free, patient-mounted, and able to steer a needle (around off-target anatomy or to correct trajectory misalignments) during the actual insertion in near real-time ( Fig. 9 ). Clinical trials by Levy et al showed that the XACT robotic system significantly decreased the duration of procedures by at least 30% and lowered the radiation dose, while maintaining targeting accuracy. 34 Correction capability under real-time imaging guidance may help overcome the challenge of requiring a high degree of expertise and/or experience for a successful and accurate procedure. A key feature is the ability to iteratively correct the trajectory at selected checkpoints during needle insertion.
Fig. 9.
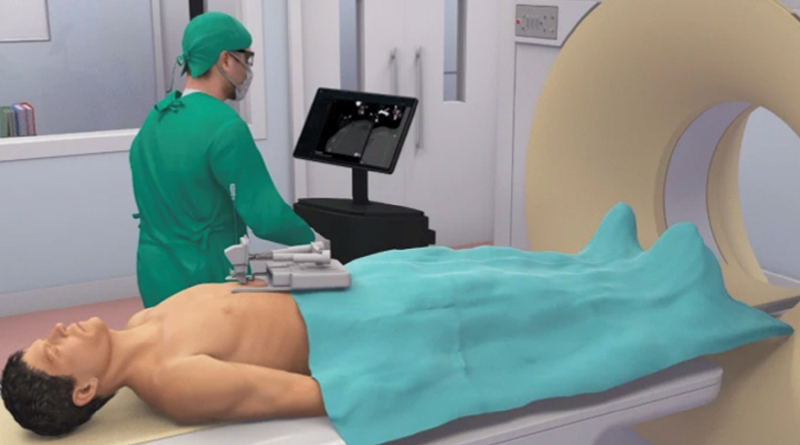
XACT patient-mounted robot. (Reprinted with permission from Levy S, Goldberg SN, Roth I, et al. Clinical evaluation of a robotic system for precise CT-guided percutaneous procedures. Abdom Radiol (NY). Published online June 19, 2021. doi: 10.1007/s00261-021-03175-9.)
Most current clinical trials represent a relatively low number of patients; so, there is a large need for greater recruitment in an expanded variety of additional organs and tissue types to increase the level of evidence for any IR robot. More randomized controlled trials are challenging to perform, but they are necessary.
CBCT
Table-mounted robots in CBCT allow for the use of the CBCT needle guidance without extension clamps and without exposing the operator's hands to the beam. The iSYS CT/CBCT system is a table-mounted CT robot with 4 DOF. Also, two parallel flat stages (a “sandwich”) translate via control by a remote joystick, resulting in less radiation during remote CT, CBCT, or fluoroscopy-guided procedures. 28 35 Such systems have also been directly integrated with CBCT needle navigation systems ( Fig. 10 ), such as X-per Guide (Philips), to allow monitoring of insertion in orthogonal entry point and progress views.
Fig. 10.
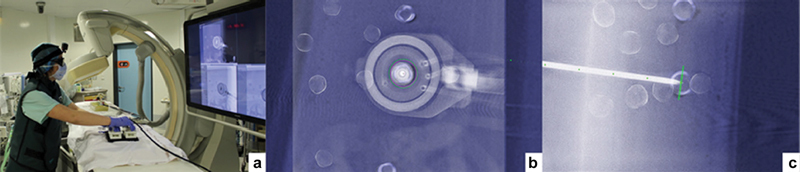
CBCT-guided robot. ( a ) Joystick controls are outside of fluoroscopic X-ray beam; ( b ) CBCT target is monitored by fluoroscopy without the operator near the X-ray beam; ( c ) view from side shows needle shaft in relation to superimposed CBCT target. (Reprinted with permission from Pfeil A, Cazzato RL, Barbé L, et al. Robotically assisted CBCT-guided needle insertions: preliminary results in a phantom model. Cardiovasc Intervent Radiol 2019;42(2):283–288.)
Magnetic Resonance Imaging
Though less established than CT, needle-based interventions can be combined with MRI to localize anatomy. MRI-guided robotic systems are most commonly used for prostate, brain, and breast cancer biopsy and ablation. One disadvantage is that it is difficult to place and maneuver a needle while the patient is in a closed-bore MRI scanner; so, the patient needs to be moved in and out of the scanner for needle-based procedures. 36 Transrectal MRI-guided prostate biopsy may be faster than when manually adjusting the needle. 37
Other Robot Applications
Vascular Robotics
Although beyond the scope of this review, endovascular robotic IR systems including Magellan and Corindus/Siemens provide automation and standardization to catheter and wire manipulations. Perhaps most exciting is the potential for machine learning or artificial intelligence-based decision-making regarding selection of manual techniques (e.g., Dotter vs. bare wire interrogation vs. jiggle and rotation). One might envision selection of a technique based on a particular set of angles and geometry combined with the knowledge of what combination worked best in past experience or procedures in similar situations.
Like percutaneous robotics for needle-based therapies, endovascular robotics might be a normalizing solution to the variability of experience, skillsets, and hand–eye coordination and the lack of standardization. Such robots might provide a standard toolkit for navigating specific anatomy or combinations of vessel maps requiring specific maneuvers or techniques. Vascular robotic systems for endovascular treatments have seen limited use until recent work reporting on the clinical merits and utility in peripheral, coronary, and cerebrovascular interventions. Several products have been FDA-cleared for years, including a 2012 FDA-clearance for peripheral coronary interventions (Corindus, now Siemens CorPath robot). 38 39 Any procedural based medical practice might be improved by additional elements of standardization, some of which may be provided by robotics (for training and practice).
Bronchoscopy
IR robotics activity pales in comparison to investments and new devices made in the much smaller field of interventional pulmonology, which has seen major investment, development, and deployment in the space of bronchial robotics and transbronchial ablation (Johnson and Johnson, Intuitive, Medtronic). Recently, reimbursed lung cancer screening in smokers may have increased the clinical need for such precision technologies for small indeterminate nodules. Endobronchial interventions will thus become more standardized with the ongoing adoption of such robotic tools, many of which are married to a partner ablation technology (such as Johnson and Johnson's NeuWave flexible endobronchial microwave with Auris's Monarch robot and radial endobronchial ultrasound). Endobronchial ablation is being developed, if not driven, by simultaneous deployment and investment in endobronchial robotic navigation.
Electromagnetic navigational biopsy combines electromagnetic navigation and CT imaging, but it has many technical difficulties, such as signal jitter and poor stability of the probe and catheter beyond the bronchoscope. The Monarch (Auris, Johnson and Johnson) relies in part on electromagnetic navigation, whereas Ion (Intuitive) uses shape-sensing technology to enhance bronchoscope precision and stability and improve localization of peripheral pulmonary lesions. Early studies with these robots had nodule localization rates of 93 to 97% with no complications. 40 41 42
Compared with endobronchial robotics, CBCT with 3D airway road mapping for navigation may keep the interventional radiologist at the table, for either percutaneous or endobronchial interventions. Philips has driven the development of 3D fluoroscopic guidance by referencing a 3D CBCT or CT road-map for the airways and target lesion (similar to CBCT EmboGuide or Ablation Planner). 40
Non-Robotic Needle Interventional Tools
Our team has investigated other less-complicated technologies to guide needle interventions which may serve the same purpose as robotics for percutaneous needle insertion. This is admittedly a very small and biased list, but these technologies include augmented reality, microelectromechanical systems, gyroscopic trackers, and smartphone or goggle-based visualization or angle selection.
AngleNav is a wireless device with a microelectromechanical tracker that can be clipped to a needle to provide a Bluetooth or audible depiction of desired needle angles to reduce error. 43 Augmented reality (AR) superimposes digital information on the physical body, improving situational awareness and enhancing image-guided interventions. Using AR for needle-guided interventions has similar goals to robotics, potentially with less cost and complexity. Ergonomics and workflow remain major hurdles for any system, robotic or otherwise. 44 An AR smartphone application ( Fig. 11 ), for example, enabled real-time needle tracking and reduced insertion errors and procedure time compared with CBCT and freehand CT-guided insertion. 45 46
Fig. 11.
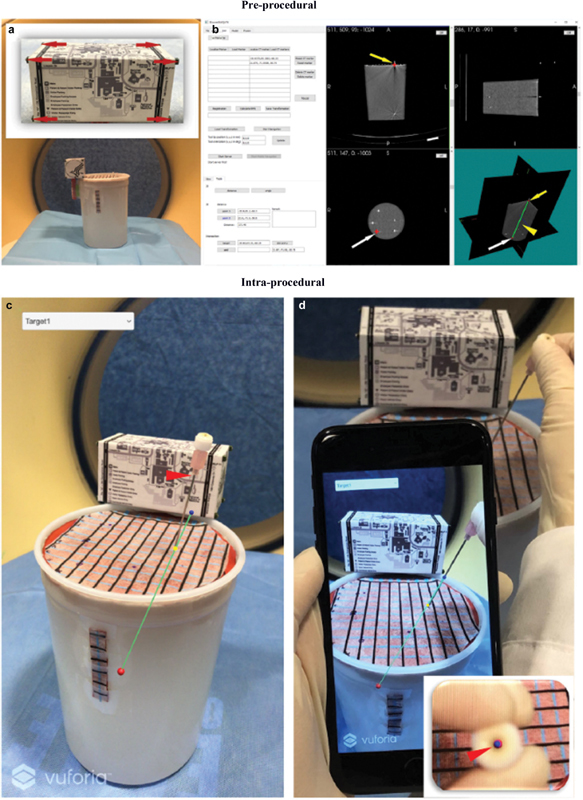
Smartphone image of an aligned needle. (Reprinted with permission from Hecht R, Li M, de Ruiter QMB, et al. Smartphone augmented reality CT-based platform for needle insertion guidance: a phantom study. Cardiovasc Intervent Radiol 2020;43(5):756–764.)
Conclusions, Challenges, and Future Questions
Although superficially attractive, IR robotics will have to prove added value with streamlined workflows and documented cost-effectiveness to meaningfully impact clinical practice of IR and IO. However, several questions remain to be addressed. What will be the smoothest interface for hardware, software, and human workflow integration? When will it help? How will it be reimbursed or where will costs be captured? Will it disrupt workflow? Is robotics needed if you have tracking or augmented reality or CBCT fusion or EM or optical tracking is available? Will the IR robot autonomously insert as well as autonavigate? Can IR robotics shorten the learning curve or enhance standardization so desperately needed for expert procedures? Will it enable more reliable precision for planned composite treatment of larger tumors? IR researchers must scientifically assess and define the value of novel technologies before other disciplines solve the clinical need via alternate methods. The studies must be new, randomized, and value-added, and not repeats of past failures. The time is now. For IR robotics, it is better to be at the table than on the menu.
Funding Statement
Funding Sources This work was supported by the Center for Interventional Oncology and the Intramural Research Program of the National Institutes of Health (NIH) by intramural NIH Grants Z01 1ZID BC011242 and CL040015. This research was also made possible through the NIH Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and contributions to the Foundation for the NIH from the Doris Duke Charitable Foundation, the American Association for Dental Research, the Colgate-Palmolive Company, and other private donors.
Disclosures
The content of this publication does not necessarily reflect the views or policies of the National Institutes of Health, the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government.
N.V. is an employee of Philips Research North America.
B.J.W. is principal investigator on the following CRADAs (Cooperative Research and Development Agreements) between NIH and related commercial partners Philips Image Guided Therapy (CRADA), Philips Research (CRADA), Philips (CRADA), Siemens (CRADA), Canon Medical (Licensing Agreement), NVIDIA (CRADA), Boston Scientific (CRADA), Celsion Corp (CRADA), as well as XACT Robotics (CRADA), and prior research agreement with Perfint Healthcare. B.J.W. is a coinventor on 50 issued patents where NIH is the assignee. B.J.W. receives royalties from the NIH-Philips patent licensing agreement on technologies related to navigation and fusion biopsy, among other topics.
References
- 1.Kassamali R H, Ladak B. The role of robotics in interventional radiology: current status. Quant Imaging Med Surg. 2015;5(03):340–343. doi: 10.3978/j.issn.2223-4292.2015.03.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tacher V, de Baere T. Robotic assistance in interventional radiology: dream or reality? Eur Radiol. 2020;30(02):925–926. doi: 10.1007/s00330-019-06541-w. [DOI] [PubMed] [Google Scholar]
- 3.Leal Ghezzi T, Campos Corleta O. 30 Years of robotic surgery. World J Surg. 2016;40(10):2550–2557. doi: 10.1007/s00268-016-3543-9. [DOI] [PubMed] [Google Scholar]
- 4.Wood B J, Yanof J H. CT-guided interventional oncology: bridging the gap between diagnosis and therapy. Kontraste (Hamburg) 2005;49(03):28–32. [Google Scholar]
- 5.Makarov D V, Li H, Lepor H, Gross C P, Blustein J. Teaching hospitals and the disconnect between technology adoption and comparative effectiveness research: the case of the surgical robot. Med Care Res Rev. 2017;74(03):369–376. doi: 10.1177/1077558716642690. [DOI] [PubMed] [Google Scholar]
- 6.Williams S B, Prado K, Hu J C. Economics of robotic surgery: does it make sense and for whom? Urol Clin North Am. 2014;41(04):591–596. doi: 10.1016/j.ucl.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 7.Koethe Y, Xu S, Velusamy G, Wood B J, Venkatesan A M. Accuracy and efficacy of percutaneous biopsy and ablation using robotic assistance under computed tomography guidance: a phantom study. Eur Radiol. 2014;24(03):723–730. doi: 10.1007/s00330-013-3056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beermann M, Lindeberg J, Engstrand J. 1000 consecutive ablation sessions in the era of computer assisted image guidance - lessons learned. Eur J Radiol Open. 2019;6:1–8. doi: 10.1016/j.ejro.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tinguely P, Frehner L, Lachenmayer A. Stereotactic image-guided microwave ablation for malignant liver tumors - a multivariable accuracy and efficacy analysis. Front Oncol. 2020;10:842. doi: 10.3389/fonc.2020.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schaible J, Pregler B, Verloh N. Improvement of the primary efficacy of microwave ablation of malignant liver tumors by using a robotic navigation system. Radiol Oncol. 2020;54(03):295–300. doi: 10.2478/raon-2020-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Citone M, Fanelli F, Falcone G, Mondaini F, Cozzi D, Miele V. A closer look to the new frontier of artificial intelligence in the percutaneous treatment of primary lesions of the liver. Med Oncol. 2020;37(06):55. doi: 10.1007/s12032-020-01380-y. [DOI] [PubMed] [Google Scholar]
- 12.Heerink W J, Ruiter S JS, Pennings J P. Robotic versus freehand needle positioning in CT-guided ablation of liver tumors: a randomized controlled trial. Radiology. 2019;290(03):826–832. doi: 10.1148/radiol.2018181698. [DOI] [PubMed] [Google Scholar]
- 13.Wood B J, Locklin J K, Viswanathan A.Technologies for guidance of radiofrequency ablation in the multimodality interventional suite of the future J Vasc Interv Radiol 200718(1, Pt 1):9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Markezana A, Goldberg S N, Kumar G. Incomplete thermal ablation of tumors promotes increased tumorigenesis. Int J Hyperthermia. 2021;38(01):263–272. doi: 10.1080/02656736.2021.1887942. [DOI] [PubMed] [Google Scholar]
- 15.Stone M J, Wood B J. Emerging local ablation techniques. Semin Intervent Radiol. 2006;23(01):85–98. doi: 10.1055/s-2006-939844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon S B, Patriciu A, Bohlman M E, Kavoussi L R, Stoianovici D. Robotically driven interventions: a method of using CT fluoroscopy without radiation exposure to the physician. Radiology. 2002;225(01):277–282. doi: 10.1148/radiol.2251011133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Putzer D, Schullian P, Braunwarth E. Integrating interventional oncology in the treatment of liver tumors. Eur Surg. 2018;50(03):117–124. doi: 10.1007/s10353-018-0521-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bale R, Schullian P, Eberle G. Stereotactic radiofrequency ablation of hepatocellular carcinoma: a histopathological study in explanted livers. Hepatology. 2019;70(03):840–850. doi: 10.1002/hep.30406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu S, Kruecker J, Turkbey B. Real-time MRI-TRUS fusion for guidance of targeted prostate biopsies. Comput Aided Surg. 2008;13(05):255–264. doi: 10.1080/10929080802364645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao R, Zhang L, Sun Y, Miao S, Chefdhotel C. A review of recent advances in registration techniques applied to minimally invasive therapy. IEEE Trans Multimed. 2013;15(05):983–1000. [Google Scholar]
- 21.Hoffmann J, Westendorff C, Leitner C, Bartz D, Reinert S. Validation of 3D-laser surface registration for image-guided cranio-maxillofacial surgery. J Craniomaxillofac Surg. 2005;33(01):13–18. doi: 10.1016/j.jcms.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Khan F R, Henderson J M. Vol. 116. Elsevier; 2013. Deep brain stimulation surgical techniques. Handbook of Clinical Neurology Brain Stimulation; pp. 27–37. [DOI] [PubMed] [Google Scholar]
- 23.Brown R A. A stereotactic head frame for use with CT body scanners. Invest Radiol. 1979;14(04):300–304. doi: 10.1097/00004424-197907000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Khadem R, Yeh C C, Sadeghi-Tehrani M. Comparative tracking error analysis of five different optical tracking systems. Comput Aided Surg. 2000;5(02):98–107. doi: 10.1002/1097-0150(2000)5:2<98::AID-IGS4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 25.Sorriento A, Porfido M B, Mazzoleni S. Optical and electromagnetic tracking systems for biomedical applications: a critical review on potentialities and limitations. IEEE Rev Biomed Eng. 2019;13:212–232. doi: 10.1109/RBME.2019.2939091. [DOI] [PubMed] [Google Scholar]
- 26.Franz A M, Haidegger T, Birkfellner W, Cleary K, Peters T M, Maier-Hein L. Electromagnetic tracking in medicine – a review of technology, validation, and applications. IEEE Trans Med Imaging. 2014;33(08):1702–1725. doi: 10.1109/TMI.2014.2321777. [DOI] [PubMed] [Google Scholar]
- 27.Geist E, Shimada K. Position error reduction in a mechanical tracking linkage for arthroscopic hip surgery. Int J CARS. 2011;6(05):693–698. doi: 10.1007/s11548-011-0555-7. [DOI] [PubMed] [Google Scholar]
- 28.Kettenbach J, Kronreif G. Robotic systems for percutaneous needle-guided interventions. Minim Invasive Ther Allied Technol. 2015;24(01):45–53. doi: 10.3109/13645706.2014.977299. [DOI] [PubMed] [Google Scholar]
- 29.Lachenmayer A, Tinguely P, Maurer M H. Stereotactic image-guided microwave ablation of hepatocellular carcinoma using a computer-assisted navigation system. Liver Int. 2019;39(10):1975–1985. doi: 10.1111/liv.14187. [DOI] [PubMed] [Google Scholar]
- 30.Schaible J, Lürken L, Wiggermann P. Primary efficacy of percutaneous microwave ablation of malignant liver tumors: comparison of stereotactic and conventional manual guidance. Sci Rep. 2020;10(01):18835. doi: 10.1038/s41598-020-75925-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guiu B, De Baère T, Noel G, Ronot M. Feasibility, safety and accuracy of a CT-guided robotic assistance for percutaneous needle placement in a swine liver model. Sci Rep. 2021;11(01):5218. doi: 10.1038/s41598-021-84878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiraki T, Kamegawa T, Matsuno T. Robotic needle insertion during computed tomography fluoroscopy-guided biopsy: prospective first-in-human feasibility trial. Eur Radiol. 2020;30(02):927–933. doi: 10.1007/s00330-019-06409-z. [DOI] [PubMed] [Google Scholar]
- 33.Komaki T, Hiraki T, Kamegawa T. Robotic CT-guided out-of-plane needle insertion: comparison of angle accuracy with manual insertion in phantom and measurement of distance accuracy in animals. Eur Radiol. 2020;30(03):1342–1349. doi: 10.1007/s00330-019-06477-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levy S, Goldberg S N, Roth I. Clinical evaluation of a robotic system for precise CT-guided percutaneous procedures. Abdom Radiol (NY) 2021 doi: 10.1007/s00261-021-03175-9. [DOI] [PubMed] [Google Scholar]
- 35.Pfeil A, Cazzato R L, Barbé L. Robotically assisted CBCT-guided needle insertions: preliminary results in a phantom model. Cardiovasc Intervent Radiol. 2019;42(02):283–288. doi: 10.1007/s00270-018-2088-8. [DOI] [PubMed] [Google Scholar]
- 36.Monfaredi R, Cleary K, Sharma K. MRI robots for needle-based interventions: systems and technology. Ann Biomed Eng. 2018;46(10):1479–1497. doi: 10.1007/s10439-018-2075-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barral M, Lefevre A, Camparo P. In-bore transrectal MRI-guided biopsy with robotic assistance in the diagnosis of prostate cancer: an analysis of 57 patients. AJR Am J Roentgenol. 2019;213(04):W171–W179. doi: 10.2214/AJR.19.21145. [DOI] [PubMed] [Google Scholar]
- 38.Britz G W, Panesar S S, Falb P, Tomas J, Desai V, Lumsden A.Neuroendovascular-specific engineering modifications to the CorPath GRX Robotic System J Neurosurg 2019; (epub ahead of print) 10.3171/2019.9.JNS192113 [DOI] [PubMed] [Google Scholar]
- 39.Mahmud E, Schmid F, Kalmar P. Feasibility and safety of robotic peripheral vascular interventions: results of the RAPID trial. JACC Cardiovasc Interv. 2016;9(19):2058–2064. doi: 10.1016/j.jcin.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 40.de Ruiter Q MB, Karanian J W, Bakhutashvili I. Endobronchial navigation guided by cone-beam CT-based augmented fluoroscopy without a bronchoscope: feasibility study in phantom and swine. J Vasc Interv Radiol. 2020;31(12):2122–2131. doi: 10.1016/j.jvir.2020.04.036. [DOI] [PubMed] [Google Scholar]
- 41.Jiang J, Chang S H, Kent A J, Geraci T C, Cerfolio R J. Current novel advances in bronchoscopy. Front Surg. 2020;7:596925. doi: 10.3389/fsurg.2020.596925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kent A J, Byrnes K A, Chang S H. State of the art: robotic bronchoscopy. Semin Thorac Cardiovasc Surg. 2020;32(04):1030–1035. doi: 10.1053/j.semtcvs.2020.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Li R, Xu S, Pritchard W F. AngleNav: MEMS tracker to facilitate CT-guided puncture. Ann Biomed Eng. 2018;46(03):452–463. doi: 10.1007/s10439-017-1968-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Park B J, Hunt S J, Martin C, III, Nadolski G J, Wood B J, Gade T P. Augmented and mixed reality: technologies for enhancing the future of IR. J Vasc Interv Radiol. 2020;31(07):1074–1082. doi: 10.1016/j.jvir.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hecht R, Li M, de Ruiter Q MB. Smartphone augmented reality CT-based platform for needle insertion guidance: a phantom study. Cardiovasc Intervent Radiol. 2020;43(05):756–764. doi: 10.1007/s00270-019-02403-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Long D J, Li M, De Ruiter Q MB. Comparison of smartphone augmented reality, smartglasses augmented reality, and 3D CBCT-guided fluoroscopy navigation for percutaneous needle insertion: a phantom study. Cardiovasc Intervent Radiol. 2021;44(05):774–781. doi: 10.1007/s00270-020-02760-7. [DOI] [PubMed] [Google Scholar]


