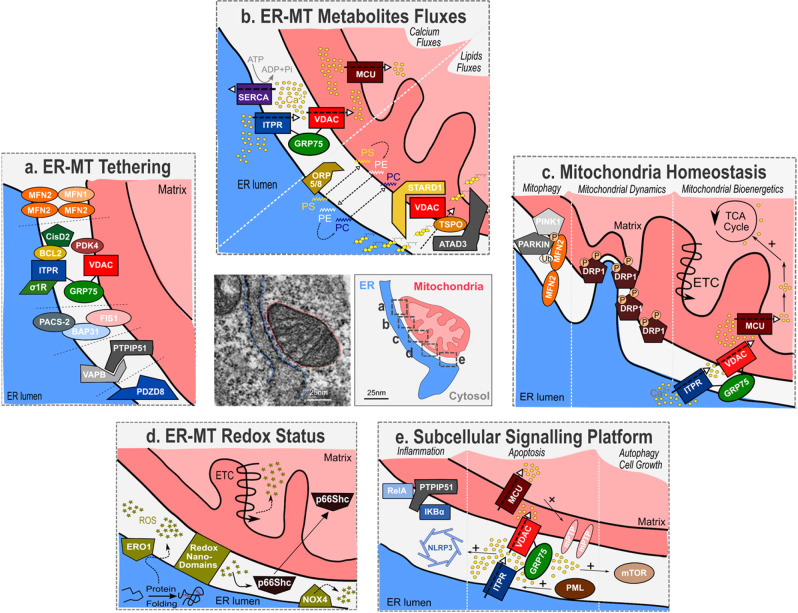
Fig. 1. Key components and associated functions of mitochondria–ER contacts.
Electron micrographs of MERCs, ER in blue and mitochondrion in red. a Multiple tethers allow the establishment of MERCs and include MFN1/MFN2 homodimers and heterodimers, ITPR-GRP75-VDAC complex, FIS1/BAP31, VAPB/PTPIP51 and PDZD8. Some MERC-associated proteins, including CisD2, PACS-2, PDK4 and SIGMAR1, interact with tethers to modulate MERCs. b MERCs are at the crossroad of calcium and lipids exchanges between ER and mitochondria. Dynamics of calcium fluxes within MERCs where ITPR, VDAC and MCU calcium channels insure transfer from ER to mitochondria and SERCA pump from cytosol to ER. Phospholipids (PS phosphatidylserine, PE phosphatidylethanolamine, PC phosphatidylcholine) are transferred trough MERCs, notably by ORP5/8 proteins, and cholesterol binds to STARD1/VDAC1/TSPO complex before being imported into the mitochondria. c MERCs regulate mitochondrial homeostasis notably through mitophagy, mitochondrial fission and mitochondrial bioenergetics. PINK1 phosphorylates MFN2, which recruits PARKIN at MERCs. PARKIN ubiquitinates MFN2 to initiate mitophagy. ER wraps mitochondria in initiation sites of fission and recruits DRP1. Calcium cation transfers from ER to mitochondria fuel some calcium-activated TCA cycle dehydrogenases and transporters to promote oxidative phosphorylation. d MERCs control redox status of ER and mitochondria. ERO1 is coupled to protein folding oxidation and generates ROS (H2O2). Redox nanodomains are formed within MERCs interface. NAPDH oxidase NOX4 produces ROS, while p66Shc senses ROS before relocalizing to mitochondria. e MERCs act as subcellular signalling platforms. Members of the NF-κB pathway, RelA and IKκBα, interact with PTPIP51. ITPR-mediated calcium release allows high local concentration of calcium and could participate in the recruitment and activation of mTOR and NLRP3. Promyelocytic Leukemia protein (PML) is found in MERCs fraction.