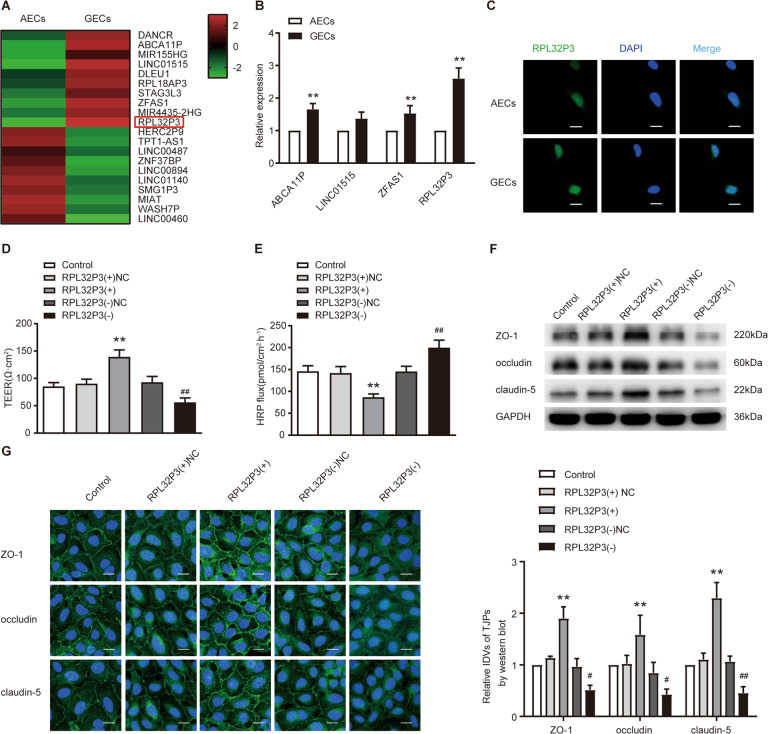
Fig. 1. Knockdown of RPL32P3 increased blood–tumor barrier (BTB) permeability in vitro.
A LncRNAs differentially expressed in astrocyte-exposed ECs (AECs) and glioma-exposed endothelial cells (GECs) were analyzed by lncRNA microarray. Red indicates high expression and green indicates low expression. B qRT-PCR was conducted to validate the selected molecules (n = 3, each group). **P < 0.01 vs. AECs group. Results were analyzed using the relative quantification (2–ΔΔCT) method. C Fluorescence in situ hybridization (FISH) was used to detect the expression and location of RPL32P3 in AECs and GECs (green, RPL32P3; blue, DAPI nuclear staining). Scale bar represents 20 μm. D, E Effects of RPL32P3 on transendothelial electric resistance (TEER) value and horseradish peroxidase (HRP) flux. Data represent mean ± SD (n = 3, each). **P < 0.01 vs. RPL32P3(+)NC group, ##P < 0.01 vs. RPL32P3 (-)NC group. F Effects of RPL32P3 on ZO-1, occludin, and claudin-5 expression levels determined by western blot. Data represent mean ± SD (n = 3, each). **P < 0.01 vs. RPL32P3 (+)NC group, #P < 0.05 vs. RPL32P3 (-)NC group, ##P < 0.01 vs. RPL32P3 (-)NC group. Western blot was analyzed in terms of integrated light density values (IDVs). G Effects of RPL32P3 on ZO-1, occludin and claudin-5 expression levels and distribution determined by immunofluorescence. Scale bar represents 30 μm.