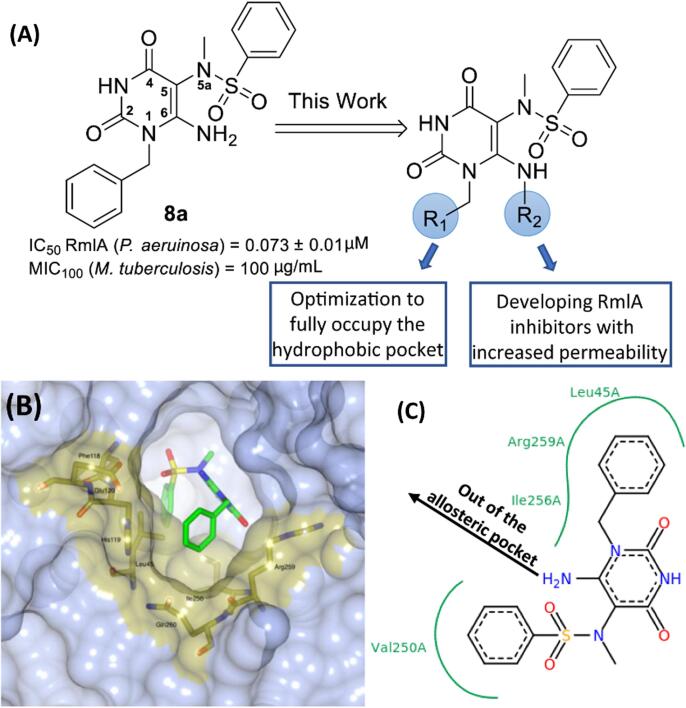
Figure 1.
A. Chemical structure and biological activity of the previously optimized inhibitor 8a. [7] IC50 against the Pseudomonas aeruginosa RmlA protein, MIC100 against Mycobacterium tuberculosis. The aims of this work were to modify the N1- and C6-NH2 positions. B. A representation of 8a bound in the allosteric site of RmlA based on our previous X-ray crystallographic analysis of the RmlA-8a complex [PDB 4ASJ]. Residues that make up the N1-substituent sub-pocket are highlighted. C. Schematic representation of pocket interactions between 8a and the enzyme showing that the C6-NH2 in 8a has the tendency to point out of the allosteric pocket into solution.