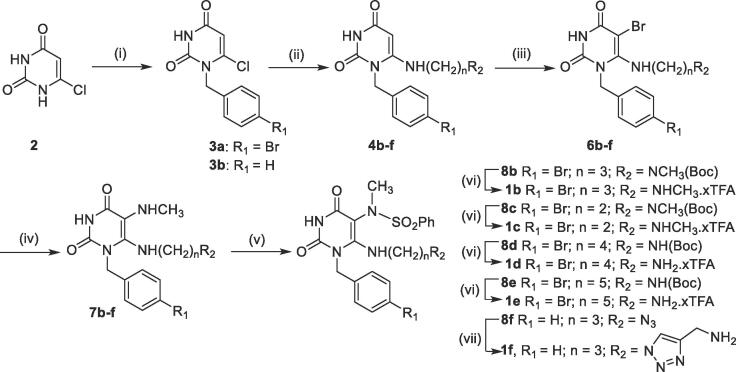
Scheme 2.
Synthesis of C6-NH2 analogues. Reagents and conditions: (i) benzyl chloride or 4-bromo-benzyl chloride, K2CO3, DMSO, 65 °C, 30 min, 3a = 38%, 3b = 45%; (ii) for 4b-4f: required amine (5b: NH2(CH2)3NCH3(Boc); 5c: NH2(CH2)2NCH3(Boc), 5d: NH2(CH2)4NHBoc, 5e: NH2(CH2)5NHBoc, 5f: NH2(CH2)3N3), EtOH, 100 °C, sealed tube, 3 hrs, 4b = 45%, 4c = 45%, 4d = 50%, 4e = 65%, 4f = 78%; (iii) N-Bromosuccinimide, MeOH, 25 °C, 10 min; 6b = 85%; 6c = 85%; 6d = 85%; 6e = 87%; 6f = 61%; (iv) 40% w.w. aq. MeNH2, 70 °C, 1 h; 7b = 56%; 7c = 42%; 7d = 67%; 7e = 80%; 7f = 94%; (v) benzenesulfonyl chloride, pyridine, DCM, 25 °C, 18 hrs; (vi) trifluoroacetic acid, DCM, 25 °C, overnight; 1b = 50%; 1c = 46%; 1d = 48%; 1e = 38%; yields are after two steps (v and vi); (vii) ascorbic acid, CuSO4·5H2O, propargylamine, tBuOH/H2O, 25 °C, 3 hrs, 1f = 10%; the yield is after two steps (v and vii).