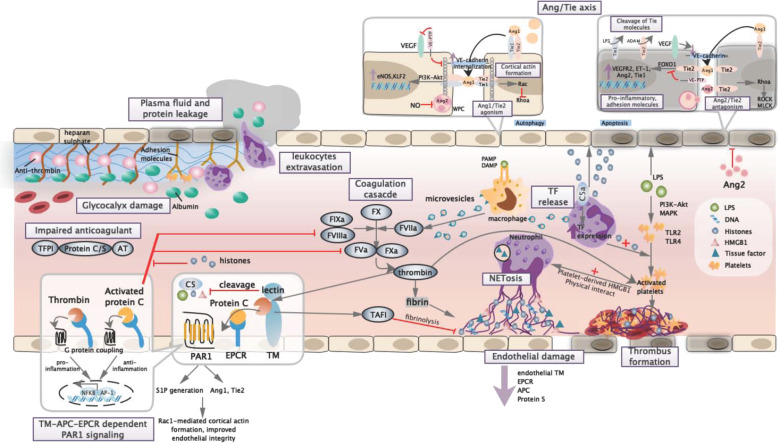
Fig. 3.
Endothelial barrier and coagulation cascades. Lined by membrane-binding proteoglycans and glycosaminoglycan side chains, dearrangement of endothelial glycocalyx result in loss of anti-thrombogenicity and exposure of adhesion molecules, which allow leukocyte adhesion, platelet recruitment, and thrombus formation. Increased vascular permeability trigger leukocyte extravasation, plasma protein leakage, and tissue edema. Under immunogenic stimuli, procoagulant tissue factors were substantially expressed in the form of microvesicles by macrophages and neutrophils, which triggered extrinsic coagulation cascade and cleavage of prothrombin. Neutrophils and platelets work reciprocally to regulate innate immune response and bacterial clearance via NETosis and platelet-mediated responses. During sepsis, NET-derived histones HMGB1 and cfDNA abundantly found serve as procoagulants that accelerate thrombus formation, while activated platelets and RBC recruitment result in thrombocytopenia and platelet-rich thrombus. In order to monitor coagulation dynamics, thrombin interacts with endothelial-bound TM to exhibit profound anti-coagulant and anti-inflammatory effects. In the presence of APC-EPCR, TM/thrombin interact with coagulation–fibrolysis system to act as negative feedback loop. APC–EPCR interaction also allow switching of PAR-1 signaling to anti-inflammatory pattern and strengthened endothelial integrity via activities mediated by S1P and Ang/Tie axis. Besides, TM/thrombin directly inactivate procoagulant alarmins to further restrict immunocoagulation. During severe infection, dysregulation of Ang/Tie axis were associated with weakened vascular stability, which include inhibition of junctional protein, destabilization of cortical actin, and increased vascular adhesion and permeability. Finally, extensive formation of inflammatory thrombus and activation of coagulation cascades result in microcirculatory dysfunction and organ injury. TM thrombomodulin, PAR-1 protease activated receptor 1, EPCR endothelial protein C receptor, S1P sphingosine-1 phosphate, TFPI tissue factor pathway inhibitor, AT antithrombin, TAFI thrombin-activatable fibrinolysis inhibitor, Rac RAS-related C3 botulinum toxin substrate, RhoA ras homolog family member A, Ang1 angiopoietin 1, Ang2 angiopoietin 2, VEGF vascular endothelial growth factor, VE-PTP vascular endothelial protein tyrosine phosphatase, WPB Weibel–Palade body, MLCK myosin light chain kinase, ROCK Rho-associated kinase, ADAM disintegrin and metalloproteinase domain-containing protein