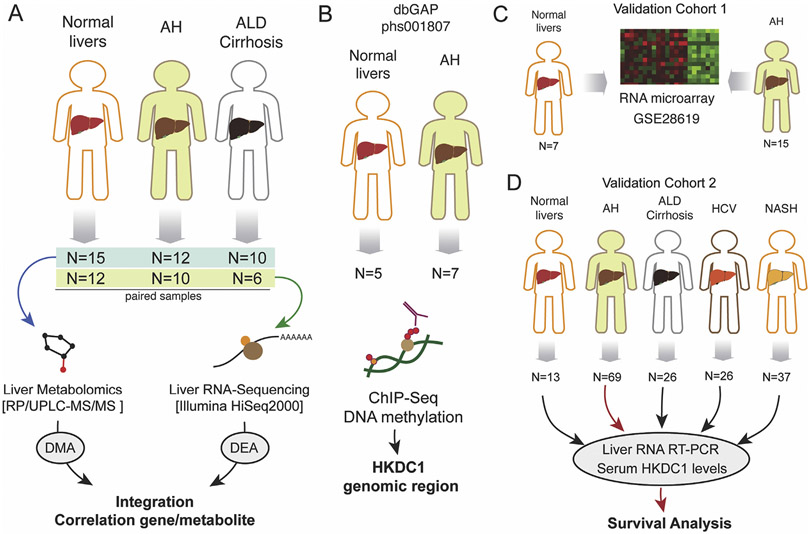
Figure 1.
Schematic work flowchart of human samples analyses. (A) Liver metabolomics and RNA-seq were performed by using a cohort of normal liver fragments from resection for liver metastasis (n = 15), livers from patients with AH (n = 12), and livers from patients with AC (n = 10). (B) Liver ChIP-seq data were retrieved from a previous study (Database of Genotypes and Phenotypes phs001807). Data from a validation cohort of patients with AH (n = 15) and control individuals (n = 7) were retrieved from previous study (GSE28619). (D) Real-time PCR validation and serum HKDC1 measurements were performed in a cohort of normal liver tissue (n = 13) and from patients with AH (n = 69), AC (n = 26), HCV-infected patients (n = 26), and patients with NASH (n = 37). dbGAP, Database of Genotypes and Phenotypes; RP/UPLC-MS/MS, reversed phase/ultraperformance liquid chromatography coupled to tandem mass spectrometry.