Figure 2.
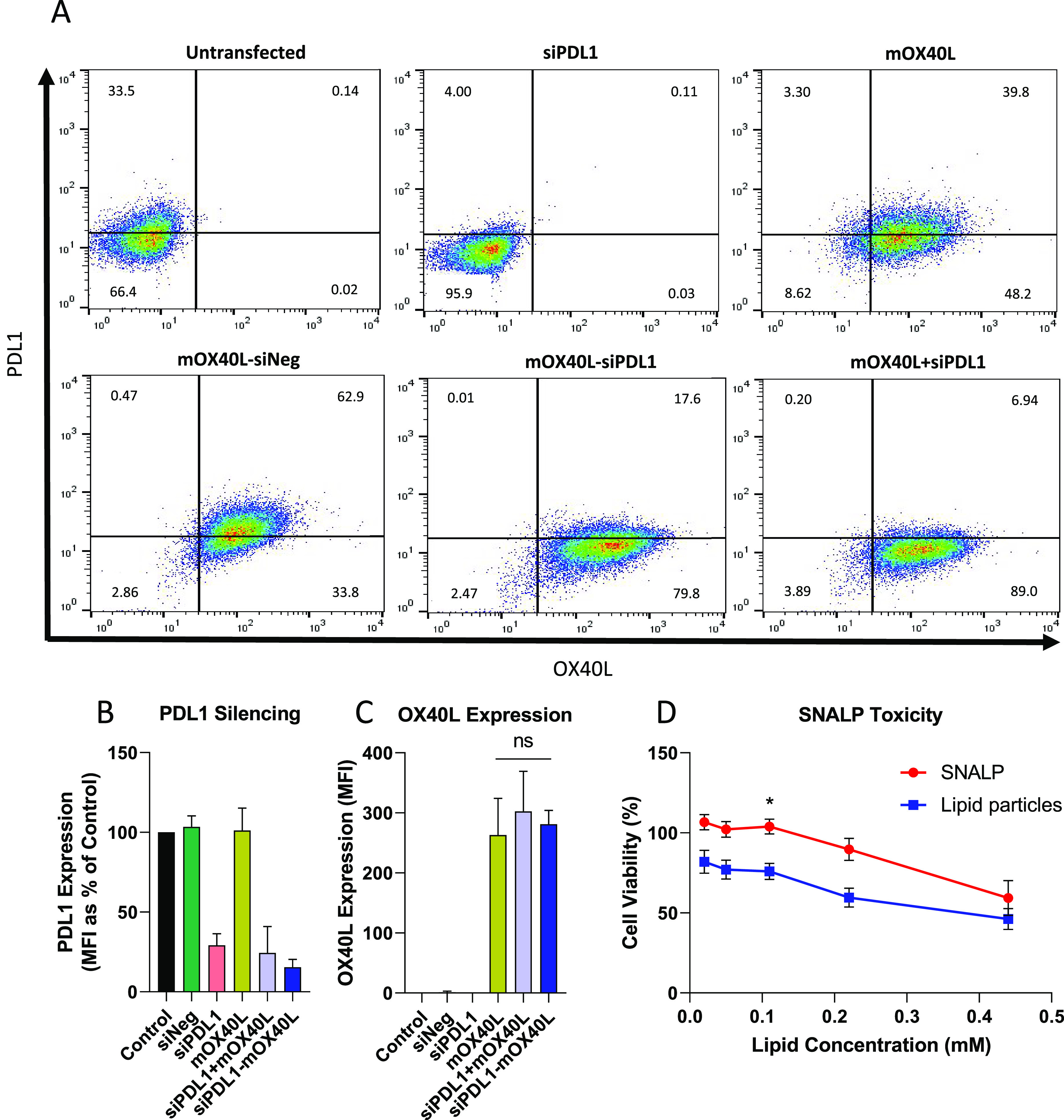
Dual-targeting SNALPs can efficiently transfect B16F10 melanoma cells in vitro and display minimal toxicity. B16F10 cells were cultured until 90% confluent before being pulsed with SNALP formulations (0.75 μg of each type of RNA) for 48 h at 37 °C. Cells were harvested and doubly stained with fluorescently labeled anti-mouse OX40L and PDL1 monoclonal antibodies. (A) Shows representative flow cytometry plots. The conditions are as follows: untransfected, siPDL1, mOX40L, mOX40L–siNeg (coformulation), mOX40L–siPDL1 (coformulation), and siPDL1 + mOX40L (mixture of two SNALPs). Quadrant gates were drawn based on isotype control antibody staining, percentage of cells in each quadrant is inset. (B) Shows the values obtained for PDL1 silencing, expressed as MFI percentage of control normalized to 100%. OX40L expression (MFI) is shown in (C). For all the graphs, error bars correspond to standard error of the mean (SEM). Significance was examined with one-way ANOVA multiple comparison test (Tukey’s); n = 3–8 repeats for each SNALP formulation. (D) To assess viability of B16F10 cells after being pulsed with SNALPs or RNA-free lipid particles an MTT assay was carried out. A 2-fold dilution series of test formulations was prepared and incubated with cells for 48 h at 37 °C. Error bars were drawn by standard error of the mean (SEM) average of n = 10, significance was tested with a two-way ANOVA: Sidak’s multiple comparison test. *,p < 0.05.
