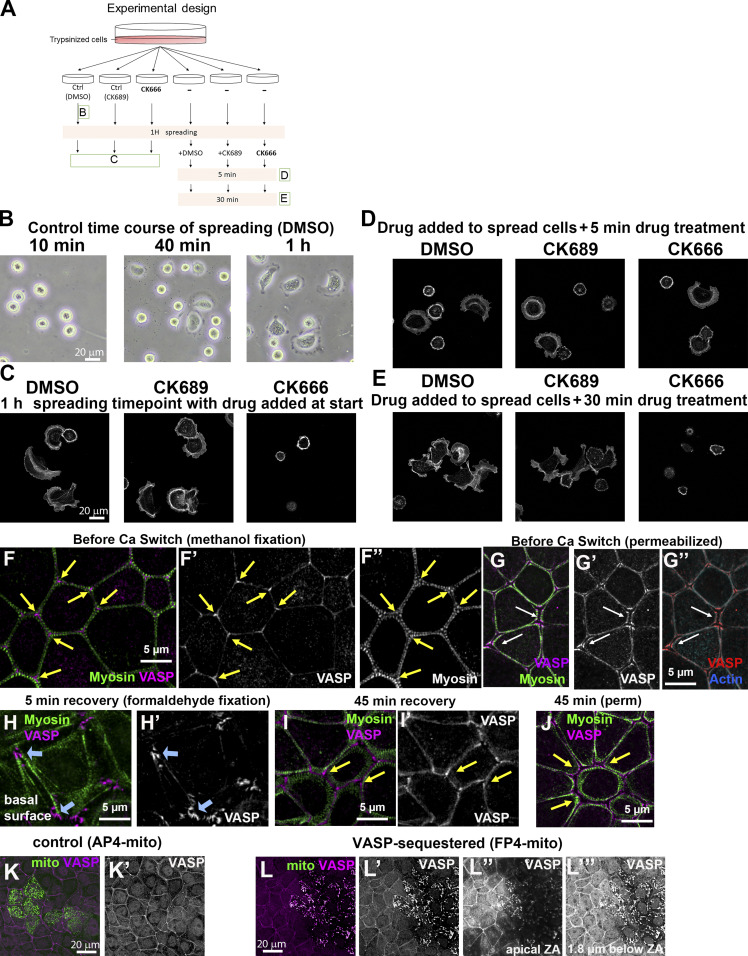
Figure S2.
Verification of the activity of CK666 for inhibiting Arp2/3 function and of AP4/FP4 constructs for sequestering Ena/VASP to mitochondria. (A–E) Verification of CK666 function. (A) Schematic diagram of the experiment. Ctrl, control. (B) Bright-field image of control cells at different spreading time points. More Arp2/3-dependent lamellipodia structures were formed the longer the cells were plated on extracellular matrix. (C–E) Phalloidin staining. (C) CK666 inhibited Arp2/3-dependent cell spreading, whereas the control (DMSO or CK689 [the inactive molecule]) did not. (D and E) Arp2/3-dependent lamellipodia disappeared in the presence of CK666. (F and G) In confluent monolayers, VASP localizes to bicellular borders and is enriched in tricellular junctions. (H) At early stages of recovery, VASP also localizes to basal focal adhesions. (I and J) As Ecad junctions start to form, VASP is detected at cell borders and tricellular junctions. (K and L) Verification of VASP-sequestering construct. (K) In cells transfected with the control construct (AP4mito), VASP remained enriched at the cortex. (L) In FP4mito-transfected cells, VASP localization to apical junctions was strongly reduced or lost, and it relocalized to internal structures we presume are mitochondria. VASP loss at cell junctions was apparent both apically (L″) and more basally (L″′).