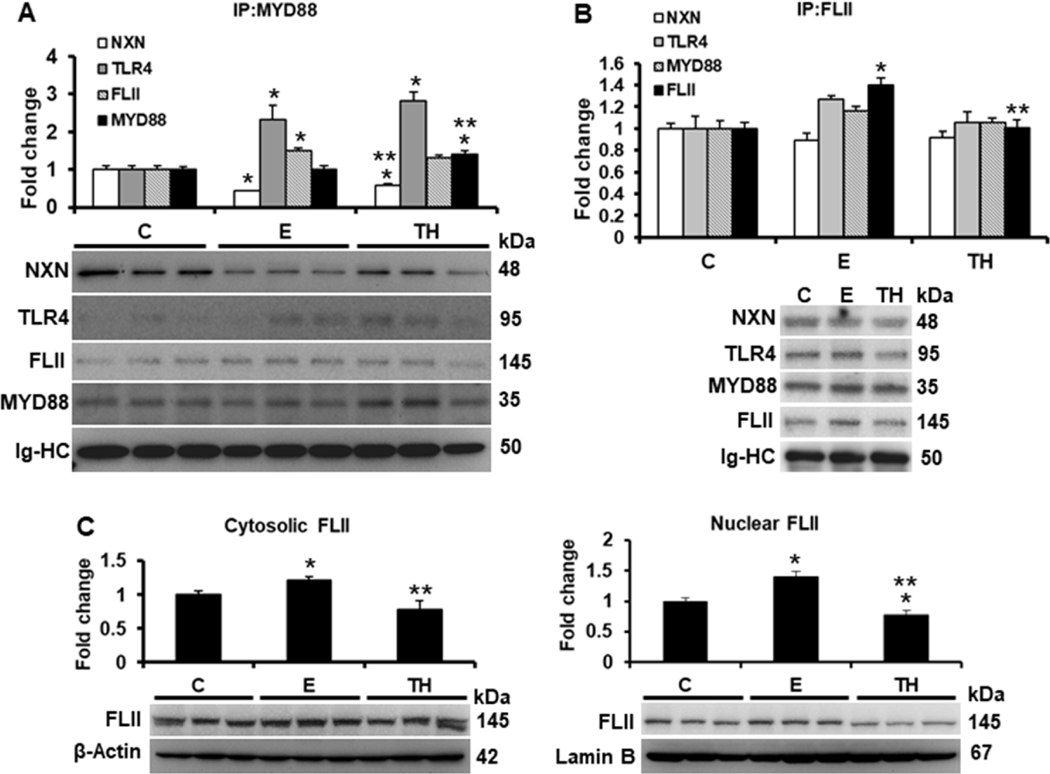
Fig. 1. Effect of ethanol on FLII/NXN/MYD88 complex and FLII status.
Total liver protein extracts were used for IP assays. (A) IP of MYD88 co-precipitated NXN, TLR4 and FLII proteins. Ethanol and TH model decreased 56% and 40% NXN/MYD88 interaction ratio, respectively, and induced 2.3 and 2.8 fold the MYD88/TLR4 interaction ratio, respectively. MYD88 precipitated level was increased 1.4 fold by TH model vs controls. (B) IP of FLII co-precipitated NXN, TLR4 and MYD88 proteins. FLII was precipitated 1.4 fold by ethanol vs controls but reverted by TH model. (C) Cytosolic and n=5 nuclear localization of FLII content. Levels of the house-keepings β-Actin and Lamin B were used to normalize cytosolic and nuclear FLII levels, respectively. All proteins were detected by western blot analyses. Densitometric analyses were used to quantify the spots. Immunoglobulin heavy chains (Ig-HC) used for IP (anti-MYD88 and anti-FLII) assays were detected and quantify to normalize precipitated protein levels. Bars values are expressed as fold change compared to controls and represent the mean ±SE. animals/group. Statistically different from *C and from **E group; p<0.05. C, Control; E, ethanol/binge; TH, ethanol/binge/LPS. n=5