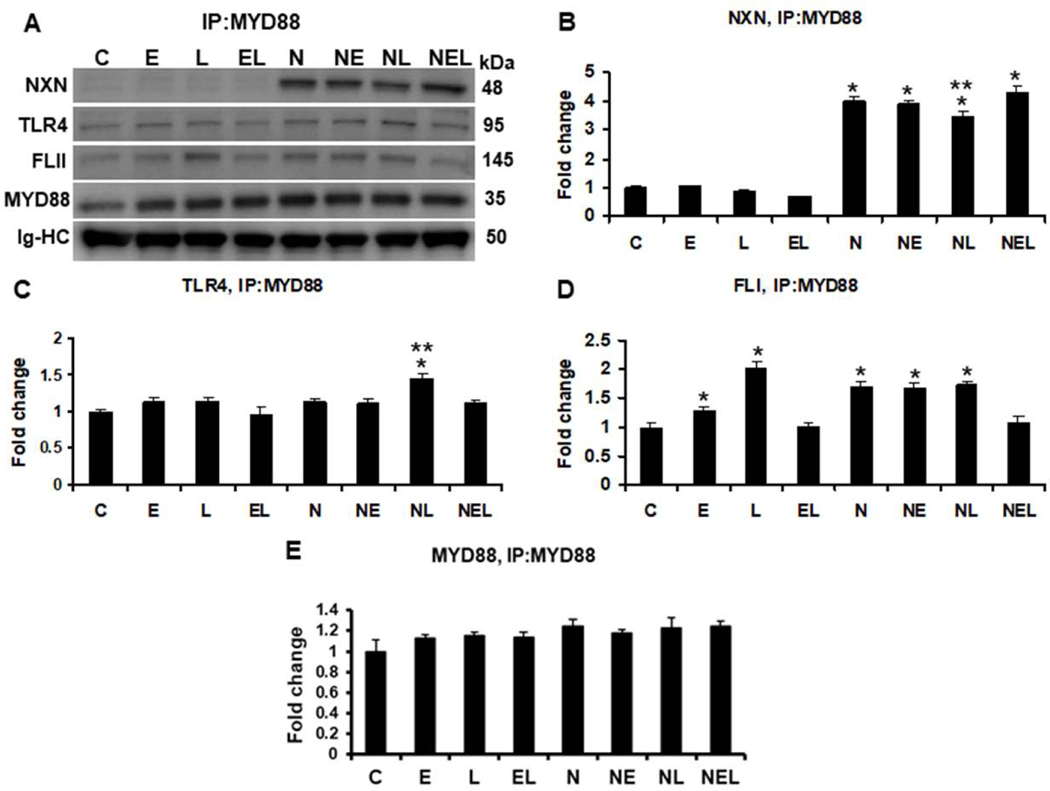
Fig. 4. IP of MYD88 and evaluation of ethanol effect on FLII/NXN/MYD88 complex in vitro.
(A) Western blot analyses were used to quantify NXN, FLII and TLR4 proteins co-immunoprecipitated with MYD88 protein. Densitometric quantification of (B) NXN, (C) TLR4, (D) FLII, and (E) MYD88 protein levels. NXN was co-precipitated more than 3 fold in NXN-transfected cells. LPS decreased 15% NXN/MYD88 interaction ratio but increased 1.4 fold MYD88/TLR4 interaction ratio in NXN overexpressing cells. Ethanol and LPS alone increased 1.3 and 2.0 fold, respectively, the MYD88/FLII binding ratio in untrasfected cells. NXN transfection itself promoted 1.7 (p<0.001) fold this binding ratio but ethanol plus LPS disrupted this interaction in untransfected cells. Total proteins from co-cultures were used for MYD88 immunoprecipitation. Immunoglobulin heavy chains (Ig-HC) used for IP (anti-MYD88 antibody) assay were detected and quantify to normalize all precipitated proteins. Bars values are expressed as fold change compared to controls and represent the mean ±SE. n=6 per group. Statistically different from *C and from **N groups; p<0.05. Groups: C, Control; E, Ethanol; L, LPS; and EL, Ethanol plus LPS, were transfected with EV and were called untransfected cells. N, NXN-transfected cells; these cells were also exposed to E, L and EL treatments and were identified as: NE, NL and NEL, respectively.