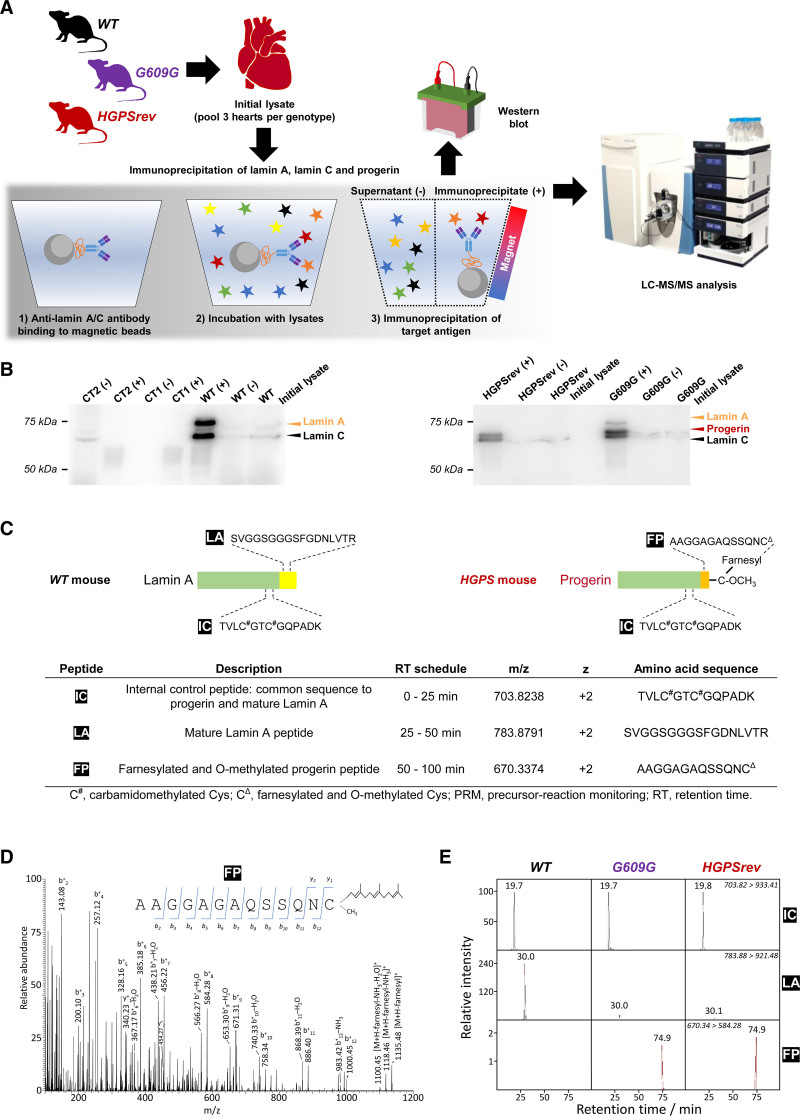
Figure 2.
Targeted precursor-reaction monitoring (PRM) analysis to examine progerin farnesylation in mouse heart lysates. A, Workflow for the LC-MS/MS analysis of proteins extracted from mouse hearts and immunoprecipitated with anti-lamin A/C antibodies that recognize lamin A, lamin C, and progerin. For each genotype, each sample was the pool of 3 hearts. WT, wild-type mice; G609G, LmnaG609G/G609G mice; HGPSrev, LmnaHGPSrev/HGPSrev mice. B, Western blots using anti-lamin A/C antibody to check the enrichment of lamin A, lamin C, and progerin in the immunoprecipitated material and supernatant (+, immunoprecipitated; –, supernatant). Controls included samples containing only beads and antibody (CT1) and only beads and protein extract (CT2). A 10-µL aliquot of each sample was loaded onto the gel; see details in Supplemental Material. C, Surrogate peptides used to detect mature lamin A and progerin: IC, internal control peptide (present in both lamin A and progerin); LA, lamin A peptide (specific for lamin A); FP, farnesylated progerin peptide (specific for progerin). D, MS2 fragmentation spectrum from FP obtained in the PRM assay. The insert shows ion ascription to the main fragment-ion series (C-terminal y-series and N-terminal b-series). E, MS/MS (tandem mass spectrometry) extracted ion chromatograms of IC, LA, and FP peptides obtained from the time-scheduled PRM assay for the detection of lamin A and progerin. The ion traces were obtained using fragment ion y+9 from IC, y+8 from LA, and b+8 from FP. LC-MS/MS indicates liquid chromatography coupled to targeted tandem mass spectrometry.