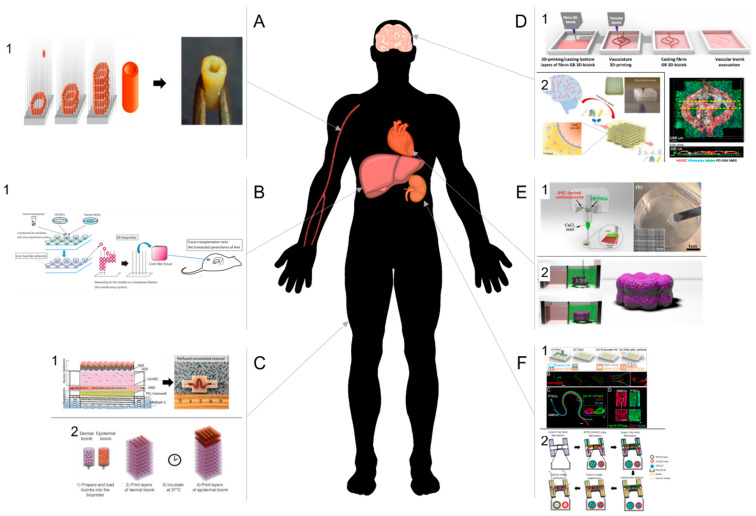
Figure 4.
3D bioprinted scaffold for tissue vascularization (A) or vascularized tissues (B–F). (A) Cardiovascular system. (1) A tubular tissue was printed from multicellular spheroids (MCS) using needle array bioprinting system (left). After fusion of the spheroids, the tubular structure can be removed from the needle array and used as a blood vessel [43]. (B) Liver. (1) A liver-like tissue was constructed from liver bud-like spheroids composed of hepatocytes, mesenchymal stem cells, and endothelial cells, on a needle array. After fusion of the spheroids, the tissue was removed and implanted onto the transected parenchyma of mouse liver [134]. (C) Skin. (1) Schematic representation (left) of a bioprinted perfusable vascularized human skin tissue composed of a hypodermal pre-adipocyte compartment containing the vascular channel and covered with a dermal layer and topped with printed primary human epidermal keratinocytes. Pictures of the perfused tissue (right) [144]. (2) Schematic representation of a bioprinted human skin equivalent composed of multiple dermal layers (dermal bioink) and two epidermal layers (keratinocytes bioink) [143]. (D) Brain. (1) Schematic representation of the printing process for the fabrication of an artificial blood-brain barrier (BBB-top). After casting a bottom layer of glioblastoma(GB)-fibrin bioink, a perfusable blood vessel was bioprinted with a sacrificial ink. The vasculature network was then covered with GB-fibrin bioink. After removal of the sacrificial ink, the vasculature system was perfused. Picture (bottom right) of the confocal imaging of the bioprinted BBB [140]. (2) Schematic representation and picture of bioprinted BBB. The vascularized neural construct was fabricated from a bioprinted interconnected blood vessels wrapped with primary pericytes, astrocytes [137]. (E) Heart. (1) Schematic representation of the bioprinting process of a heart patch composed of one layer of iPSC-derived cardiomyocytes and one layer of HUVECs (left). Picture of the patch after printing (right) [67]. (2) High cell-density cardiac fibrosis model printed from spheroids within self-healing hydrogels [125]. (F) Kidney. 3D bioprinted proximal tubule (PT). (1) Top—Schematic representation printing process of a vascularized proximal tubule (PT). Both PT and vascular channels are printed with a Pluronic-based sacrificial ink and covered with a gleatin-fibrinogen gel. After removal of the sacrificial ink, cells can be added and cultured in the lumen (top). Picture of the fabrication process; Middle—Fluorescence images of the PT (green) and the vascular (red) channel; Bottom—confocal microscopy images of both channels [148]. (3) PT and blood vessel mixed with decellularized kidney extracellular matrix (KdECM) were printed in coaxial channels to build a 3D vascularized renal proximal tubule-on-a-chip [149].