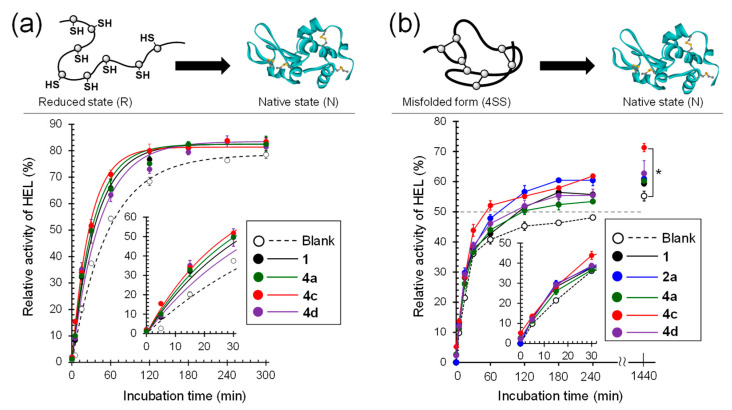
Figure 6.
Comparison of the rates of oxidative folding of RHEL and refolding of 4SS. Selected data are shown, and all other data for 1-Gly-Xaa (series 4) are shown in supporting information (Figure S1). Data are shown as means ± SEM (n = 3). (a) Enzymatic activity recovered during the oxidative folding of RHEL. The reaction conditions were [RHEL]0 = 10 μM, [GSH]0 = 1.0 mM, [GSSG]0 = 0.20 mM, [catalyst] = 0 or 20 μM, 37 °C, and pH 7.5 in the presence of 1 M urea. Circles and lines represent experimental data and simulations, respectively, which are drawn using the apparent rate constants (k). (b) Enzymatic activity recovered during the refolding of 4SS. The reaction conditions were [4SS]0 = 10 μM, [GSH]0 = 1.0 mM, [catalyst] = 0 or 20 μM, 37 °C, and pH 7.5 in the presence of 1 M urea.