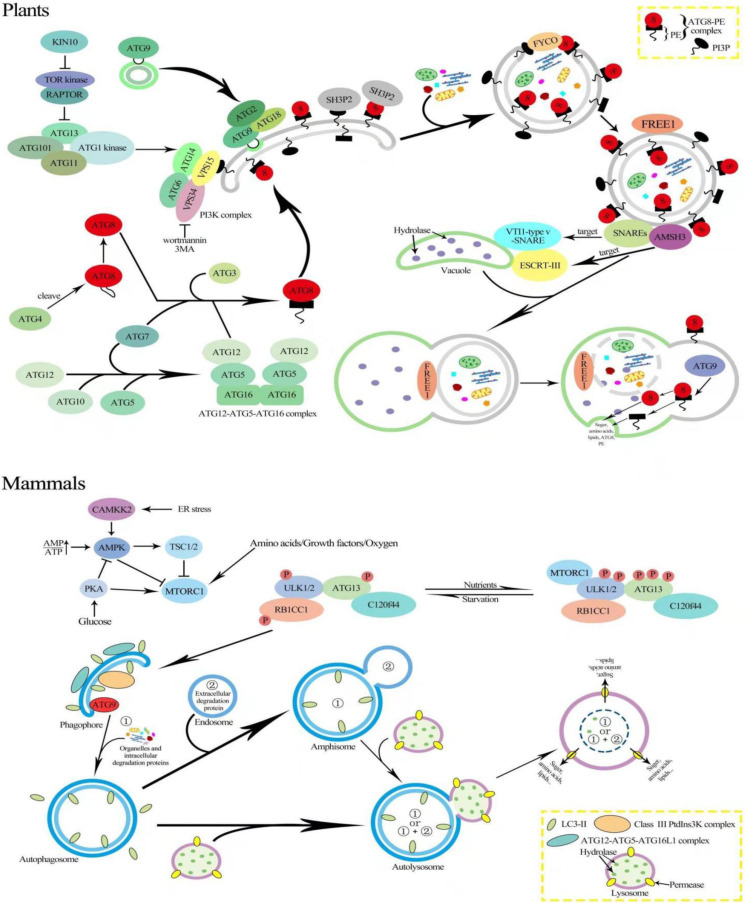
Figure 1.
Schematic illustration of autophagy regulation in plants and animals. Autophagy is activated by inhibiting TOR and is blocked when TOR is overexpressed. Autophagy is triggered by the formation of an active complex between ATG13, ATG1, ATG11, and ATG101, as well as ATG11 and ATG101, which activates autophagy. Autophagosome development comprises membrane delivery, nucleation, expansion, and closure of the phagophore. ATG9 is employed in the transport of lipids to the expanding phagophore, together with ATG2 and ATG18. PI3P decoration is generated by the VPS34 lipid kinase complex, which is followed by ATG8 conjugation to PE. Initially, ATG8 is matured by ATG4 cleaving of its C-terminal and conjugating it to PE by E2-like ATG3 and the E3-like ATG12–ATG5–ATG16 complex. For phagophore expansion, ATG8–PE binds to the autophagosomal membrane. Sealed ATG8- and PI3P-decorated autophagosomes are transported to the vacuole with the help of FYCO (FYVE and coiled-coil domain-containing) proteins that bind the autophagosome to the microtubule transport machinery. With the aid of ARP2/3 (NAP1), ESCRT (CFS1, CHMP1, FREE1, and VPS2.1), and exocyst (EXO70B1) components, SNARE-mediated fusion of autophagosomes with the tonoplast releases autophagic bodies into the vacuole. Following that, vacuolar hydrolases degrade the vesicles. Model of Ulk1 regulation by AMPK and mTORC1 in response to glucose signals. Left: when glucose is sufficient, AMPK is inactive and mTORC1 is active. The active mTORC1 phosphorylates Ulk1 on Ser 757 to prevent Ulk1 interaction with and activation by AMPK. When cellular energy level is limited, AMPK is activated and mTORC1 is inhibited by AMPK through the phosphorylation of TSC2 and Raptor. The induction complex consists of ULK1/2, ATG13, RB1CC1, and C12orf44. Under nutrient-rich conditions, MTORC1 associates with the complex and inactivates ULK1/2 and ATG13 through phosphorylation. During starvation, MTORC1 dissociates from the complex, and ATG13 and ULK1/2 become partially dephosphorylated by yet-unidentified phosphatases, allowing the complex to induce macroautophagy. RB1CC1/FIP200 and C12orf44/ATG101 are also associated with the induction complex and are essential for macroautophagy. RB1CC1/FIP200 may be the ortholog of yeast Atg17, whereas the function of C12orf44/ATG101 is not known. A signal transduction event regulated by the TOR kinase leads to the following: (1) the induction of autophagy—a membrane from an unknown source sequesters cytosol and/or organelles resulting in the formation of a double-membrane vesicle termed an autophagosome; (2) on completion—the autophagosome docks with the lysosome or vacuole. Fusion of the autophagosome outer membrane with the vacuole releases the inner vesicle into the vacuole lumen. The inner vesicle is termed an autophagic body. Breakdown within the vacuole allows the recycling of the degraded autophagic body and its hydrolyzed cargo (amino acids, fatty acids, sugars, and nucleotides).