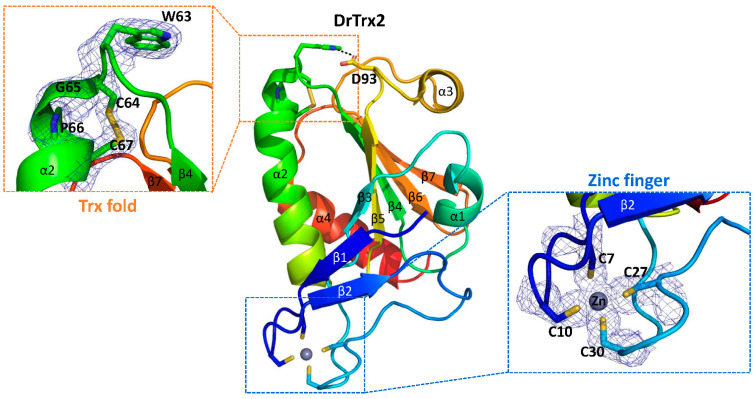
Figure 5.
Conserved cysteine residues in DrTrx2. A cartoon representation of the full-length structure of DrTrx2 is provided in a different orientation compared to Figure 4. The molecule is colored progressively, from blue at the N-terminus to red at the C-terminus. The amino acid residues in the active site 63WCGPC67 motif, the N-terminal zinc binding site, and Asp93, which makes a hydrogen bond (dotted black line) with Trp63, are shown as sticks. Dotted rectangles indicate enlarged views of the active site 63WCGPC67 motif (orange) and the N-terminal zinc binding site (blue). The zinc ion is shown as a gray sphere. The final maximum-likelihood weighted 2Fo-Fc electron density map of 63WCGPC67 and four cysteine residues coordinated with zinc ion contoured at 1σ are also presented as mesh.