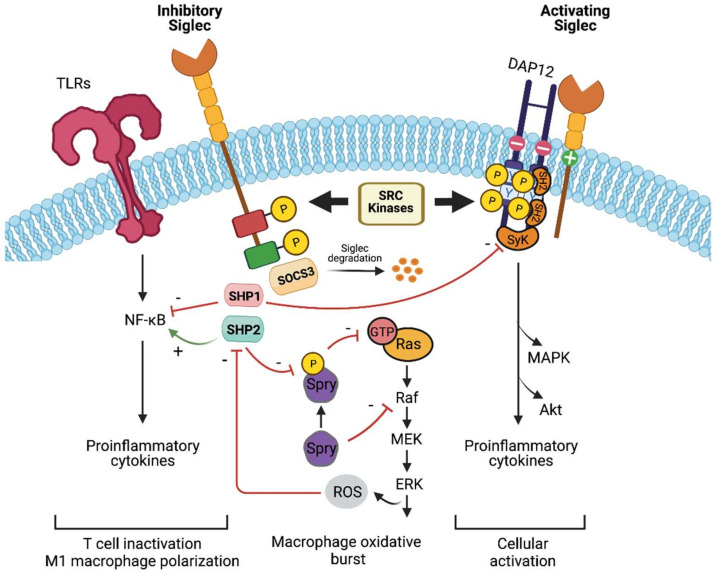
Figure 3.
Siglec signaling and immune modulatory effect. Upon sialic acid binding, SRC kinases can phosphorylate tyrosine residues found in the cytosolic ITIM/ ITAMs. Within the inhibitory Siglecs, SHP-2 is recruited to the phosphotyrosines and can inhibit Sprouty (Spry). The inhibition of Spry promotes the activation of Ras-ERK signaling and subsequent ERK and Akt-dependent production of reactive oxygen species (ROS) [182,183]. ROS in sepsis can inactivate SHP-2, resulting in endothelial activation [184]. Additionally, SHP-2 can activate the NK-κB pathway, leading to inflammation and cellular immune responses [185]. On the other hand, SHP-1 inhibits the NK-κB pathway and controls cytokine production through the JAK-STAT pathway [164,185]. SHP-1 is associated with T cell inactivation, whereas SHP-2 is associated with the activation of M1 macrophage phenotype [166,186]. Suppressor of cytokine signaling 3 (SOCS3) competes with both SHP-1/-2 for phosphorylated ITIMs, thereby inhibiting signaling and resulting in accelerated proteosomal degradation of both Siglec-3 and SOCS3 [176]. Next, within the inhibitory Siglecs, DAP12 associates with the positively charged residue found in the transmembrane of inhibitory Siglecs. Subsequently, Syk bearing tandem SH2 domains binds to the phosphorylated ITAM-containing DAP12 [187], and results in the downstream generation of second messenger, elevated intracellular calcium, and the activation of MAPK/Akt pathways. Notably, Syk can be inhibited by SHP-1 [188], and thus modulate the activating and inhibitory signaling of Siglecs.