Abstract
Oral diseases or poor oral hygiene have close connections with systemic inflammatory reaction, which is one of major mechanism in the development of chronic kidney disease (CKD). We conducted a research assuming that better oral hygiene care would be negatively related with the risk of developing new-onset CKD.
From 2003 to 2004, a total of 158,495 participants from the Korean national health insurance data sharing service which provides health screening data including variables as age, sex, vascular risk factors, medication information, indicators regarding oral hygiene, and laboratory results. The diagnosis of CKD and vascular risk factors were defined according to the International Statistical Classification of Diseases and Related Health Problems codes-10th revision. The follow-up period for the study subject was until the occurrence of CKD, until death, or Dec 31, 2015.
Approximately 13.3% of the participants suffered from periodontal disease, and 40.7% brushed their teeth at least three times a day. With a median of 11.6 (interquartile range 11.3–12.2) years’ follow-up, the cohort included 3223 cases of incident CKD. The 10-year incidence rate for CKD was 1.80%. In multivariable analysis with adjustment for age, sex, demographics, vascular risk factors, blood pressure, and blood laboratory results, frequent tooth brushing (≥3 times a day) was negatively related to occurrence of CKD (hazard ratio: 0.90, 95% confidence interval [0.83–0.99], P = .043, P value for trend = .043).Participants with improved oral hygiene (≥3 times a day) have showed less risk of CKD. Additional interventional studies are in need to establish causative relationship between oral hygiene and risk of CKD.
Keywords: chronic, epidemiology, oral hygiene, periodontitis, renal insufficiency
1. Introduction
Chronic kidney disease (CKD) is one of most significant health problems facing the world. Approximately 8%∼10% of the general population is considered to have mild-to-moderately decreased renal function, and the prevalence of CKD continues to increase.[1–3] Moreover, CKD is a major cause of cardiovascular disease, cerebrovascular disease and death.[4] Thus, it is necessary to investigate and identify risk factors associated with CKD development.
Meanwhile, oral disease, for instance tooth loss and periodontal disease, is common in general populations worldwide.[5] The tooth loss or periodontal disease can cause focal inflammation of surrounding tissues such as gingiva, ligaments of periodontal organ, and alveolar bone.[6] In addition, these tooth loss or periodontal disease may also cause systemic inflammation.[6] Also, there are other oral hygiene indicators including tooth brushing frequency and professional scaling. Tooth brushing is associated with good oral hygiene.[7] Regular brushing can reduce periodontal disease and oral disease.[8,9] Since oral hygiene has an effect on systemic health, frequent tooth brushing actually reduced the risk for development of diabetes, heart failure, and atrial fibrillation in general population studies.[10,11]
The inflammatory reactions are a major contributor to the development of CKD.[12] In a population-based cohort study, systemic inflammatory biomarkers were associated with increased risk of developing incident CKD.[13] Because periodontal disease may be associated with microbially triggered chronic inflammatory disease, these systemic inflammatory response by oral infection and release of inflammatory cytokines and their mediators can affect renal dysfunction.[12,14] In addition, tooth brushing can reduce inflammation in the oral cavity and the resulting systemic inflammatory reactions, and vice versa.[8,9,15] In contrary, bacteraemia can be transiently induced by tooth brushing.[16] Therefore, it can be presumed that the inflammatory reaction caused by periodontitis or poor oral hygiene management may affect the development of CKD. Furthermore, CKD patients often develop oral diseases such as periodontitis or gingivitis,[17,18] and patients with hemodialysis are more prone to severe periodontal diseases.[19] But most studies that have shown associations between oral health and CKD are case–control or cross-sectional studies on specific populations.
We assumed that good oral hygiene care may be negatively related with the risk of CKD development in a general population-based longitudinal study. We aimed to clarify the relationship between oral hygiene indicators and CKD in a nationwide general population-based cohort.
2. Methods
2.1. Study design and data sources
The design of our study was a retrospective cohort study. In our study, 171,829 participants older than 40 years who participated in oral health examination were selected from the National Health Insurance System-National Health Screening Cohort (NHIS-HEALS) between 2003 and 2004.[10,20] The National Health Insurance Service is the only public insurance agency in Korea, with most Koreans covered by the insurance. All insured persons are entitled to a biennial standardized medical examination that includes routine medical check-up, lifestyle survey and oral health check-up. The cohort data consist of demographic data, salary level, alcohol drinking, smoking status, exercise status, blood pressure, body weight and height, concomitant diseases including cardiovascular risk factors (hypertension, diabetes mellitus, dyslipidemia, any malignancy, atrial fibrillation, and heart failure), and oral health status.[11]
NHIS provides researchers with a random sampling database of health screening including oral health examination and medical information. Among the 171,829 participants, after excluding 8094 participants with missing value for at least one variable in oral health check-up, 4924 participants with missing data for routine medical examination and 316 participants with proteinuria and/or known renal disease (International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10); N17–19) before the index date (the day of the health examination), 158,495 participants were finally included in our study (Supplementary figure 1). The Institutional Review Board of Ewha Womans University College of Medicine (2020–08–018) approved the analysis and provided a consent waiver, as the data were anonymized and freely accessible by the NHIS for study purposes.
2.2. Definitions
The oral health screening program comprises a self-assessment survey that includes questions regarding dental visits for any reason within one year, tooth brushing frequency and professional scaling within one year. The periodontal disease was defined as if a revised version of ICD-10 codes used in Korea (acute and chronic periodontitis, periodontosis, and other or unspecified periodontal disease [K052∼K056, respectively]) were claimed at least two times by a dentist, or when participants were performed therapy for periodontitis with relevant revised version of ICD-10 codes (K052∼K056) by a dentist.[21–23] Oral hygiene behavior was trichotomized according to the tooth brushing frequency into 0∼1, 2, and ≥3 per day. Dental clinic visit and professional scaling were both dichotomized into zero and more than once a year. The number of tooth loss cases in the oral health screening program was confirmed by the dentists. Information on alcohol consumption and smoking status was acquired through self-assessment survey. The body mass index was considered as the participant's weight divided by the square of the participant's height. Regular exercise was defined as intense physical activity performed more than 20 minutes at least once a week. Body mass index and laboratory results were applied with the most recent dataset. Detailed definitions of comorbid diseases are described in the supplementary material. (Supplementary method, which demonstrates definition of variables).
The primary outcome was the occurrence of CKD (ICD-10 codes: N18.1, N18.2, N18.3, N18.4, N18.5, and N18.9), which was defined as the disease entity being noted >2 times. The included participants were followed to the occurrence of CKD, death, or December 31, 2015. The diagnosis for CKD was used and confirmed in previous studies.[24] The accuracy of diagnosis for the risk factors including the CKD using the ICD-10 codes was 85.0% to 94.1% in previous studies using the NHIS-HEALS dataset.[24,25] Although the accuracy of diagnosis of vascular risk factors using ICD-10 codes was 85.0%∼94.1% in previous studies using the NHIS-HEALS dataset and overall sensitivity of diagnosis for malignancy using ICD-10 codes was previously found to be 92.8%, because we relied on ICD coding of the principal diagnosis, we were not able to validate individual ICD codes.[11,26,27]
2.3. Statistical analysis
Categorical variables are described as numbers (percent) and continuous variables are noted as means ± standard deviations. To test the normality of the data, we used Shapiro-Wilk normality test for continuous variables and each P value was >0.05. Relationships between continuous variables were investigated by independent t test or analysis of variance test, and relationships between the categorical variables were assessed by χ2 test. The impact of oral hygiene indicators and behaviors on the development of CKD were evaluated using Kaplan–Meier analysis with log-rank tests. The Cox proportional hazard model was utilized to identify associations between interested variables and CKD occurrence. We identified the hazard ratios (HRs) and 95% confidence intervals (CIs). To confirm the association with each oral hygiene indicators, multivariable regression analysis were performed in after adjustments for age and sex (model 1); age, sex, earning levels, consumption of alcohol, status of smoking, physical activity, body mass index, vascular risk factors, malignancy history, atrial fibrillation, and heart failure (model 2); variables adjusted in model 2, systolic blood pressure, and blood laboratory examination results (model 3). The linear trend was estimated by number of teeth loss as a continuous variable (none of teeth loss as 0, 1–7 teeth loss as 1, 8–14 teeth loss as 2, 15–21 teeth loss as 3 and ≥22 teeth loss as 4) and number of tooth brushings as a continuous variable (0–1 time per day as 0, 2 times as 1, and ≥3 times as 2).[11,23] Subgroup analyses were investigated according to demographic data and vascular risk comorbidities. The heterogeneity between oral hygiene indicators and subgroups was estimated with a 2-sided Wald test with adjusting variables used in model 3. Since the dataset consists of a random sampling of general population, sample size calculation to obtain statistical significance could not be performed. Statistical analysis was performed with SAS software (version 9.2, SAS Institute, Cary, NC). The P values with <.05 were considered as statistically significant.
3. Results
The average age of the participants was 52.3 years; 60.9% had male sex, 39.0% was diagnosed with hypertension, 8.8% was diagnosed with diabetes mellitus, and 24.9% were current smokers. About 13.3% and 42.5% of participants had periodontal disease and had visited dental clinic, respectively. On the self-assessment survey report, 40.7% of the participants brushed their teeth at least 3 times a day, and 24.0% of the participants had professional scaling (Table 1).
Table 1.
Baseline characteristics of the study population.
| Characteristics | Total |
| No. of subjects | 158,495 |
| Age, y | 52.3 ± 8.8 |
| Male sex | 96,601 (60.9) |
| Earning levels | |
| Fifth quintile (highest) | 62,850 (39.7) |
| Fourth quintile | 32,287 (20.4) |
| Third quintile | 21,813 (13.8) |
| Second quintile | 19,780 (12.5) |
| First quintile (lowest) | 21,522 (13.6) |
| Covered by medical aid | 243 (0.2) |
| Consumption of alcohol | 74,670 (47.1) |
| Status of smoking | |
| None | 103,180 (65.1) |
| Former smoker | 15,850 (10.0) |
| Current smoker | 39,511 (24.9) |
| Physical activity | 15,375 (9.7) |
| Anthropometric measurements | |
| Body mass index, kg/m2 | 23.9 ± 2.9 |
| Systolic blood pressure, mmHg | 126.2 ± 17.1 |
| Diastolic blood pressure, mmHg | 79.2 ± 11.1 |
| Comorbidities | |
| Hypertension | 61,767 (39.0) |
| Diabetes mellitus | 13,894 (8.8) |
| Dyslipidaemia | 25,145 (15.9) |
| History of malignancy | 12,423 (7.8) |
| Atrial fibrillation | 647 (0.4) |
| Heart failure | 1834 (1.2) |
| Laboratory findings | |
| Total cholesterol, mmol/L | 5.126 ± 0.938 |
| Fasting blood glucose level, mmol/L | 5.367 ± 1.576 |
| Aspartate aminotransferase, U/L | 26.0 ± 16.0 |
| Alanine aminotransferase, U/L | 25.4 ± 20.0 |
| Gamma glutamyl transferase, U/L | 38.0 ± 52.5 |
| Oral health status | |
| Periodontal disease | 21,109 (13.3) |
| Tooth loss | |
| 0 | 120,656 (76.1) |
| 1–7 | 33,877 (21.4) |
| 8–14 | 2352 (1.5) |
| 15–21 | 765 (0.5) |
| ≥22 | 845 (0.5) |
| Oral hygiene care | |
| Dental clinic visit | 67,306 (42.5) |
| Tooth brushing frequency (times/day) | |
| 0–1 | 23,314 (14.7) |
| 2 | 70,710 (44.6) |
| ≥3 | 64,471 (40.7) |
| Professional scaling | 38,059 (24.0) |
Data are expressed as the mean ± SD, or n (%).
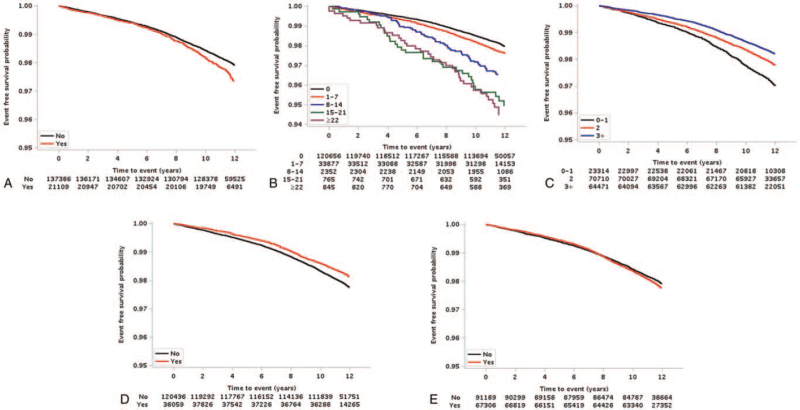
During median 11.6 years (11.3–12.2 years for interquartile range, from 2 weeks to 12.9 years) follow-up, the cohort included 3223 cases of new-onset CKD. The estimated 10-year event rate was 1.80% for CKD. The Kaplan–Meier survival curves for CKD are presented in Figure 1 according to oral hygiene indicators. Periodontal disease and a large number of tooth loss were positively related with an elevated risk of CKD occurrence (P < .001). Meanwhile, the risk of CKD had a negative correlation as the frequency of tooth brushing increased and professional scaling (P < .001, for both parameters).
Figure 1.
Kaplan–Meier survival curves associated with oral health indicators and for risk of chronic kidney disease occurrence. The Kaplan–Meier curve shows that risk of chronic kidney disease occurrence depends on periodontal disease (A) (P < .001) and tooth loss (B) (P < .001) were positively associated with an increased risk of chronic kidney disease occurrence. Meanwhile, the risk of chronic kidney disease had a negative correlation with increased tooth brushing frequency (C) (P < .001) and professional scaling (D) (P < .001), however, dental clinic visit (E) does not (P = .067).
In multivariable models, periodontal disease was positively related with the occurrence of CKD (HR [1.12]; 95% CI:1.02–1.24; P = .016) in model 1, however, this association was diminished in model 2 (HR [1.05]; 95% CI: 0.96–1.16; P = .288) and model 3 (HR [0.96]; 95% CI: 0.96–1.17; P = .223). Increased number of tooth brushings (≥3 times a day) was inversely associated with development of CKD in model 1 (HR [0.86]; 95% CI: 0.81–0.99: P = .030, P value for trend = .021), model 2 (HR [0.89]; 95% CI: 0.82–0.99; P = .040, P value for trend = .041) and in model 3 (HR [0.90]; 95% CI:0.83–0.99; P = .043, P value for trend = .043) (Table 2). Although relationship of other oral health indicators (dental clinic visit, professional scaling and tooth loss) with occurrence of CKD was noted in univariable analysis, these associations were weakened after adjusting confounding factors in multivariable analysis (Table 2).
Table 2.
Risk of chronic kidney disease according to oral health disease and oral hygiene care.
| Unadjusted model | Multivariable adjusted (1) | Multivariable adjusted (2) | Multivariable adjusted (3) | ||||||||
| No. of subjects | Number of events | Event rate % (95% CI) | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | HR (95% CI) | P | |
| Periodontal disease | |||||||||||
| No | 13,7386 | 2727 | 1.57 (1.50–1.64) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| Yes | 21,109 | 496 | 1.83 (1.65–2.01) | 1.21 (1.10–1.33) | <.001 | 1.12 (1.02–1.24) | .016 | 1.05 (0.96–1.16) | .288 | 1.06 (0.96–1.17) | .223 |
| Tooth loss | |||||||||||
| 0 | 12,0656 | 2295 | 1.49 (1.42–1.56) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| 1–7 | 33,877 | 777 | 1.81 (1.66–1.95) | 1.22 (1.12–1.32) | <.001 | 1.04 (0.96–1.12) | .387 | 1.00 (0.92–1.08) | .971 | 0.99 (0.92–1.08) | .901 |
| 8–14 | 2352 | 77 | 2.82 (2.12–3.52) | 1.83 (1.45–2.29) | <.001 | 0.93 (0.74–1.17) | .524 | 0.95 (0.76–1.20) | .683 | 0.94 (0.75–1.18) | .602 |
| 15–21 | 765 | 35 | 4.21 (2.71–5.72) | 2.65 (1.90–3.70) | <.001 | 1.17 (0.83–1.63) | .367 | 1.18 (0.84–1.65) | .332 | 1.17 (0.84–1.64) | .358 |
| ≥22 | 845 | 39 | 4.25 (2.78–5.73) | 2.82 (2.06–3.88) | <.001 | 0.96 (0.70–1.32) | .807 | 1.03 (0.75–1.42) | .848 | 1.02 (0.74–1.41) | .889 |
| P for trend∗ | <.001 | .699 | 0.773 | .878 | |||||||
| Dental clinic visit | |||||||||||
| No | 91,189 | 1800 | 1.58 (1.49–1.66) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| Yes | 67,306 | 1423 | 1.64 (1.54–1.74) | 1.07 (1.00–1.14) | .066 | 1.10 (1.00–1.18) | .093 | 1.09 (1.00–1.17) | .059 | 1.10 (1.00–1.17) | .058 |
| Tooth brushing frequency (times/day) | |||||||||||
| 0–1 | 23,314 | 643 | 2.23 (2.03–2.42) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| 2 | 70,710 | 1485 | 1.65 (1.55–1.74) | 0.74 (0.67–0.81) | <.001 | 0.91 (0.83–1.00) | .048 | 0.90 (0.82–0.99) | .024 | 0.91 (0.83–1.01) | .058 |
| ≥3 | 64,471 | 1095 | 1.34 (1.25–1.43) | 0.60 (0.55–0.67) | <.001 | 0.86 (0.81–0.99) | .030 | 0.89 (0.82–0.99) | .040 | 0.90 (0.83–0.99) | .043 |
| P for trend∗ | <.001 | .021 | .041 | .043 | |||||||
| Professional scaling | |||||||||||
| No | 12,0436 | 2547 | 1.67 (1.60–1.74) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| Yes | 38,059 | 676 | 1.40 (1.28–1.52) | 0.84 (0.77–0.91) | <.001 | 0.99 (0.91–1.08) | .836 | 0.99 (0.91–1.08) | .817 | 0.99 (0.91–1.08) | .879 |
Event rates are reported as 10-year event rates (%).
Multivariable model (1) was adjusted for age and sex.
Multivariable model (2) was adjusted for age, sex, earning levels, consumption of alcohol, status of smoking, physical activity, body mass index, vascular risk factors, malignancy history, atrial fibrillation and heart failure.
Multivariable model (3) was adjusted for the variables in model 2 as well as systolic blood pressure, fasting blood glucose level, aspartate aminotransferase, alanine aminotransferase, and gamma glutamyl transferase.
CI indicates confidence interval; HR, hazard ratio.
Trend test for hazard ratios.
In the subgroup analysis, no statistical interaction was observed between tooth brushing frequency and occurrence of CKD regarding demographics or comorbidities (age, sex, alcohol drinking, smoking status, exercise, and vascular risk factors) in multivariable models 2 and 3 (Supplementary table 1 and 2, Tables that illustrate the subgroup analysis). In contrast, participants with body mass index ≥25 and tooth brushing frequency at least 3 times a day were more strongly associated with occurrence of CKD (P for interaction < .05) (Supplementary table 1 and 2, Tables that illustrate the subgroup analysis).
4. Discussion
The main finding of our research is that increased tooth brushing frequency, especially above 3 times a day, may attenuate the risk of CKD occurrence. The cross-sectional relationship between oral hygiene and CKD has been noted previously. In a case–control study, participants with CKD had a higher frequency of periodontal diseases such as gum bleeding, abnormal taste, burning mouth, xerostomia, and candidiasis compared to those of age- and sex-matched participants without CKD.[28] In patients receiving peritoneal dialysis, frequent tooth brushing was associated with a lower incidence of peritoneal dialysis-related peritonitis.[29] Moreover, tooth brushing twice daily was a favorable factor against periodontal disease in maintenance hemodialysis patients.[30] In a longitudinal setting, poor oral hygiene was found to be positively related to the risk of cardiovascular disease in a European population.[31] In addition, better oral hygiene care, including frequent tooth brushing and professional scaling, were negatively related with the risk of future cardiovascular events in healthy Asian adults.[23] Our findings are consistent with these previous studies and also suggest further evidence on the association between better oral hygiene behavior and decreased risk for CKD occurrence.
In our study, periodontal disease was positively, and professional scaling was negatively associated with CKD occurrence in the univariable analysis. Periodontal disease was a significant association factor for CKD in a Korean general population survey study (odds ratio: 1.39).[32] Analyses of 12,947 adults representing the US population showed a positive association between CKD and periodontal disease (odds ratio: 1.60).[33] In Asian general population cohort study, periodontal disease was related with new-onset diabetes mellitus, which is a major risk factor for the occurrence of CKD.[10] However, after adjustment of several significant confounding factors, the associations of periodontal disease and professional scaling with the occurrence of CKD became insignificant. These results may suggest that factors other than the periodontal disease and professional scaling may underlie these associations with CKD. Nevertheless, based on our findings that frequent tooth brushing reduces the risk of CKD, along with the results of the univariable analysis, oral hygiene improvement is likely to attenuate the risk of CKD occurrence.
In our subgroup analysis, relatively obese participant with frequent tooth brushing more significantly associated with occurrence of CKD compared with non-obese participants. Obesity is an important risk factor in the development and progress of CKD.[34] Moreover, the tooth brushing frequency and utilization of oral products for dental health was lower in individuals with obesity.[35] Although it is hard to give clear evidence, it is plausible that obese participants may be not frequently brush teeth, and these parts may have influenced the more frequent occurrence of CKD, particularly in obese participants.
Although our research could not exactly identify the mechanism on which the relationship between oral hygiene and occurrence of CKD, the following hypotheses may explain this association. Periodontitis are closely related to systemic inflammation,[36] one of the main causes of CKD. Gum ulcers in the periodontal pockets allow oral bacteria to invade the systemic circulation.[37] Oral bacterial pathogens are known to cause alterations in the gut microbiota, which leads to indirect induction of nephrotoxic toxins or a nephrotoxic microbiota composition.[37] Furthermore, systemic inflammation caused by poor oral hygiene has been associated with CKD. Serum inflammatory markers including tumor necrosis factor-α, interleukin-2, C-reactive protein, and interleukin-6 have been positively correlated to the risk of CKD and were increased in patients with poor oral hygiene.[38–42]
Meanwhile, regular and frequent tooth brushings are known to lower the risk of periodontitis and reduce oral inflammatory reactions.[9,15] In previous studies, frequent tooth brushing was associated with decreased gingival pocket bacterial diversity,[43] and decreased inflammatory biomarkers.[31] In addition, tooth brushing improves oral hygiene trough reduction of dental plaque,[44] and by reducing diversity of bacterial biofilm in oral cavity.[45,46] As good oral hygiene can be maintained by daily tooth brushing, our finding that frequent tooth brushing decreases the risk of developing CKD may be ascribed to these mechanisms.
Our study has several limitations. First, our dataset had not considered genetic components. The genetic components of each participant might be different, even if they were of the same race, which might affect oral hygiene. Second, because our dataset consists only of Asian populations, the conclusion of this research cannot be generalized to other ethnicities. Third, although we used the definition of CKD based on previous research, the causes of CKD could not be identified in our study. Fourth, although dentists have objectively determined whether periodontal disease exists, it was not confirmed by unbiased methods such as dental x-rays. Fifth, number of tooth brushing per day and professional scaling were self-evaluated, so there is a possibility of recall bias. Sixth, the NHIS-HEALS cohort dataset does not provide detailed data about the participant's education or occupation. Therefore, income levels were the only adjustment variables that reflected socioeconomic status. Seventh, because our study design is a retrospective cohort study despite its longitudinal nature, selection bias may exist. Finally, direct causal relationships cannot be concluded, as association of frequent tooth brushing with CKD can also be explained by the fact that people with better oral hygiene may have healthier eating habits.
In conclusion, participants with improved oral hygiene (≥3 times a day) have shown less risk of CKD. Further investigations are in need to investigate the effect of oral hygiene care for CKD.
Author contributions
Conceptualization, Investigation, Methodology, Resources, and Writings - draft: Y.C. and T-J.S.
Data curation, Formal analysis, and Visualization: J.S.L.
Writing – review & editing: H.G.W., D.R.R. and J.W.K
Project administration, Supervision, and Funding acquisition: T-J.S.
Conceptualization: Yoonkyung Chang, Tae-Jin Song.
Data curation: Ji Sung Lee.
Formal analysis: Ji Sung Lee.
Funding acquisition: Tae-Jin Song.
Investigation: Yoonkyung Chang, Tae-Jin Song.
Methodology: Yoonkyung Chang, Tae-Jin Song.
Project administration: Tae-Jin Song.
Resources: Yoonkyung Chang, Tae-Jin Song.
Supervision: Tae-Jin Song.
Visualization: Ji Sung Lee.
Writing – original draft: Yoonkyung Chang, Tae-Jin Song.
Writing – review & editing: Ho Geol Woo, Dong-Ryeol Ryu, Jin-Woo Kim, Tae-Jin Song.
Supplementary Material
Footnotes
Abbreviations: CIs = confidence intervals, CKD = chronic kidney disease, HRs = hazard ratios, ICD-10 = International Statistical Classification of Diseases and Related Health Problems, Tenth Revision, NHIS-HEALS = the National Health Insurance System-National Health Screening Cohort.
How to cite this article: Chang Y, Lee JS, Woo HG, Ryu DR, Kim JW, Song TJ. Improved oral hygiene care and chronic kidney disease occurrence: A nationwide population-based retrospective cohort study. Medicine. 2021;100:47(e27845).
Data availability statement: Data requests are available at the website of the National Health Insurance Sharing Service. Because NHIS data are public data, these are accessible to anyone. To use NHIS data, a completed request form, research proposal, and approval form of the institutional review board must be submitted to and approved by the NHIS review committee. Currently, the use of NHIS data is permitted only for Korean researchers.
The authors report no conflicts of interest.
This project was supported by a grant from the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (2021R1F1A1048113 to T-JS, 2021R1I1A1A01059868 to YC). The funding source had no role in the design, conduct, or reporting of the study.
The data that support the findings of this study are available from a third party, but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are available from the authors upon reasonable request and with permission of the third party.
Supplemental digital content is available for this article.
References
- [1].Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the united states. JAMA 2007;298:2038–47. [DOI] [PubMed] [Google Scholar]
- [2].Park JI, Baek H, Jung HH. Prevalence of chronic kidney disease in Korea: The Korean national health and nutritional examination survey 2011-2013. J Korean Med Sci 2016;31:915–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chang Y, Ryu S, Choi Y, et al. Metabolically healthy obesity and development of chronic kidney disease: a cohort study. Ann Intern Med 2016;164:305–12. [DOI] [PubMed] [Google Scholar]
- [4].Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet 2017;389:1238–52. [DOI] [PubMed] [Google Scholar]
- [5].Kassebaum NJ, Smith AGC, Bernabe E, et al. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 countries, 1990-2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res 2017;96:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- [7].Wiegand A, Schlueter N. The role of oral hygiene: does toothbrushing harm? Monogr Oral Sci 2014;25:215–9. [DOI] [PubMed] [Google Scholar]
- [8].Schiffner U, Bahr M, Effenberger S. Plaque and gingivitis in the elderly: a randomized, single-blind clinical trial on the outcome of intensified mechanical or antibacterial oral hygiene measures. J Clin Periodontol 2007;34:1068–73. [DOI] [PubMed] [Google Scholar]
- [9].Poklepovic T, Worthington HV, Johnson TM, et al. Interdental brushing for the prevention and control of periodontal diseases and dental caries in adults. Cochrane Database Syst Rev 2013;Cd009857. [DOI] [PubMed] [Google Scholar]
- [10].Chang Y, Lee JS, Lee KJ, Woo HG, Song TJ. Improved oral hygiene is associated with decreased risk of new-onset diabetes: a nationwide population-based cohort study. Diabetologia 2020;63:924–33. [DOI] [PubMed] [Google Scholar]
- [11].Chang Y, Woo HG, Park J, Lee JS, Song TJ. Improved oral hygiene care is associated with decreased risk of occurrence for atrial fibrillation and heart failure: a nationwide population-based cohort study. Eur J Prev Cardiol 2020;27:1835–45. [DOI] [PubMed] [Google Scholar]
- [12].Akchurin OM, Kaskel F. Update on inflammation in chronic kidney disease. Blood Purif 2015;39:84–92. [DOI] [PubMed] [Google Scholar]
- [13].Shankar A, Sun L, Klein BE, et al. Markers of inflammation predict the long-term risk of developing chronic kidney disease: a population-based cohort study. Kidney Int 2011;80:1231–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hickey NA, Shalamanova L, Whitehead KA, Dempsey-Hibbert N, van der Gast C, Taylor RL. Exploring the putative interactions between chronic kidney disease and chronic periodontitis 2020;46:61–77. [DOI] [PubMed] [Google Scholar]
- [15].Sconyers JR, Crawford JJ, Moriarty JD. Relationship of bacteremia to toothbrushing in patients with periodontitis. J Am Dent Assoc 1973;87:616–22. [DOI] [PubMed] [Google Scholar]
- [16].Forner L, Larsen T, Kilian M, Holmstrup P. Incidence of bacteremia after chewing, tooth brushing and scaling in individuals with periodontal inflammation. J Clin Periodontol 2006;33:401–7. [DOI] [PubMed] [Google Scholar]
- [17].Summers SA, Tilakaratne WM, Fortune F, Ashman N. Renal disease and the mouth. Am J Med 2007;120:568–73. [DOI] [PubMed] [Google Scholar]
- [18].Klassen JT, Krasko BM. The dental health status of dialysis patients. J Can Dent Assoc 2002;68:34–8. [PubMed] [Google Scholar]
- [19].Ausavarungnirun R, Wisetsin S, Rongkiettechakorn N, Chaichalermsak S, Udompol U, Rattanasompattikul M. Association of dental and periodontal disease with chronic kidney disease in patients of a single, tertiary care centre in Thailand. BMJ Open 2016;6:e011836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seong SC, Kim YY, Park SK, et al. Cohort profile: The national health insurance service-national health screening cohort (nhis-heals) in Korea. BMJ Open 2017;7:e016640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Lee JH, Choi JK, Jeong SN, Choi SH. Charlson comorbidity index as a predictor of periodontal disease in elderly participants. J Periodontal Implant Sci 2018;48:92–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Lee JH, Lee JS, Park JY, et al. Association of lifestyle-related comorbidities with periodontitis: a nationwide cohort study in Korea. Medicine (Baltimore) 2015;94:e1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Park SY, Kim SH, Kang SH, et al. Improved oral hygiene care attenuates the cardiovascular risk of oral health disease: a population-based study from Korea. Eur Heart J 2019;40:1138–45. [DOI] [PubMed] [Google Scholar]
- [24].Choe WS, Choi EK, Han KD, et al. Association of metabolic syndrome and chronic kidney disease with atrial fibrillation: a nationwide population-based study in Korea. Diabetes Res Clin Pract 2019;148:14–22. [DOI] [PubMed] [Google Scholar]
- [25].Soon-Og B, Gilwon K. A comparative study of the disease codes between Korean national health insurance claims and Korean national hospital discharge in-depth injury survey. Health Policy Manag 2014;24:322–9. [Google Scholar]
- [26].Seo HJ, Oh IH, Yoon SJ. A comparison of the cancer incidence rates between the national cancer registry and insurance claims data in Korea. Asian Pac J Cancer Prev 2012;13:6163–8. [DOI] [PubMed] [Google Scholar]
- [27].Lee SJ, Im JS, Choi JS. Sensitivity of medical insurance claims data using population based cancer registry data. J Korean Soc Med Inform 2002;8:35–40. [Google Scholar]
- [28].Oyetola EO, Owotade FJ, Agbelusi GA, Fatusi OA, Sanusi AA. Oral findings in chronic kidney disease: implications for management in developing countries. BMC Oral Health 2015;15:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Oka H, Yamada S, Kamimura T, et al. Better oral hygiene habits are associated with a lower incidence of peritoneal dialysis-related peritonitis. Ther Apher Dial 2019;23:187–94. [DOI] [PubMed] [Google Scholar]
- [30].Hou Y, Wang X, Zhang CX, et al. Risk factors of periodontal disease in maintenance hemodialysis patients. Medicine (Baltimore) 2017;96:e7892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].de Oliveira C, Watt R, Hamer M. Toothbrushing, inflammation, and risk of cardiovascular disease: results from Scottish health survey. BMJ 2010;340:c2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Han SS, Shin N, Lee SM, Lee H, Kim DK, Kim YS. Correlation between periodontitis and chronic kidney disease in Korean adults. Kidney Res Clin Pract 2013;32:164–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Fisher MA, Taylor GW, Shelton BJ, et al. Periodontal disease and other nontraditional risk factors for CKD. Am J Kidney Dis 2008;51:45–52. [DOI] [PubMed] [Google Scholar]
- [34].Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney Int 2008;73:19–33. [DOI] [PubMed] [Google Scholar]
- [35].Park JB, Nam GE, Han K, Ko Y, Park YG. Obesity in relation to oral health behaviors: an analysis of the Korea national health and nutrition examination survey 2008-2010. Exp Ther Med 2016;12:3093–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Cullinan MP, Seymour GJ. Periodontal disease and systemic illness: will the evidence ever be enough? Periodontology 2000 2013;62:271–86. [DOI] [PubMed] [Google Scholar]
- [37].Hajishengallis G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015;15:30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Loos BG. Systemic markers of inflammation in periodontitis. J Periodontol 2005;76:2106–15. [DOI] [PubMed] [Google Scholar]
- [39].Kooman JP, Dekker MJ, Usvyat LA, et al. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol 2017;313:F938–50. [DOI] [PubMed] [Google Scholar]
- [40].Wing MR, Patel SS, Ramezani A, Raj DS. Gut microbiome in chronic kidney disease. Exp Physiol 2016;101:471–7. [DOI] [PubMed] [Google Scholar]
- [41].Lee BT, Ahmed FA, Hamm LL, et al. Association of c-reactive protein, tumor necrosis factor-alpha, and interleukin-6 with chronic kidney disease. BMC Nephrol 2015;16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Yeo ES, Hwang JY, Park JE, Choi YJ, Huh KB, Kim WY. Tumor necrosis factor (TNF-alpha) and C-reactive protein (CRP) are positively associated with the risk of chronic kidney disease in patients with type 2 diabetes. Yonsei Med J 2010;51:519–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pyysalo MJ, Mishra PP, Sundström K, Lehtimäki T, Karhunen PJ, Pessi T. Increased tooth brushing frequency is associated with reduced gingival pocket bacterial diversity in patients with intracranial aneurysms. PeerJ 2019;7:e6316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Patil SP, Patil PB, Kashetty MV. Effectiveness of different tooth brushing techniques on the removal of dental plaque in 6-8 year old children of Gulbarga. J Int Soc Prevent Communit Dent 2014;4:113–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Naumova EA, Weber L, Pankratz V, Czenskowski V, Arnold WH. Bacterial viability in oral biofilm after tooth brushing with amine fluoride or sodium fluoride. Arch Oral Biol 2019;97:91–6. [DOI] [PubMed] [Google Scholar]
- [46].Laine ML, Loos BG, Crielaard W. Gene polymorphisms in chronic periodontitis. Int J Dent 2010;2010:324719. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.