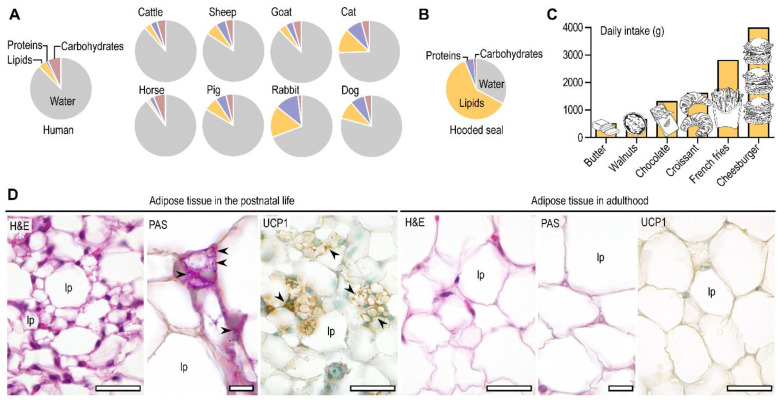
Figure 1.
Fat intake and adipose tissue during postnatal life. (A) Composition of the breast milk in humans and various mammals. (B) Composition of breast milk in the hooded seal Cystophora cristata, which is recognized as the most fat-rich breast milk among mammals [20]. (C) Illustration of the daily fat intake of a breastfed newborn human infant extrapolated to a human adult. The graph shows the weight of various food items which provide the daily fat intake of a breastfed infant (e.g., fat content of ~1250g chocolate is equivalent with the daily fat intake of a breastfed infant). (D) Histology of the subcutaneous adipose tissue after birth and in adulthood in the mouse [17]. Notably, the infant adipose tissue is rich in multilocular (“beige”) adipocytes, contains PAS+ glycogen granules, and expresses UCP1 protein. These traits allow an active lipid catabolism, lipid synthesis from glycogen, and heat generation from fatty acid oxidation. In turn, the adult adipose tissue is built up from unilocular adipocytes, which do not store glycogen, nor express UCP1. Postnatal development of the subcutaneous adipose tissue is associated with a shift from lipid catabolism and thermogenesis to lipid storage and thermal insulation [17]. H&E, hematoxylin and eosin; PAS, periodic acid Schiff (staining glycogen); UCP1, uncoupling protein 1 (immunohistochemistry); lp, lipid droplet. Arrowheads label glycogen granules and UCP1+ cell clusters. Scale bar 50 μm.