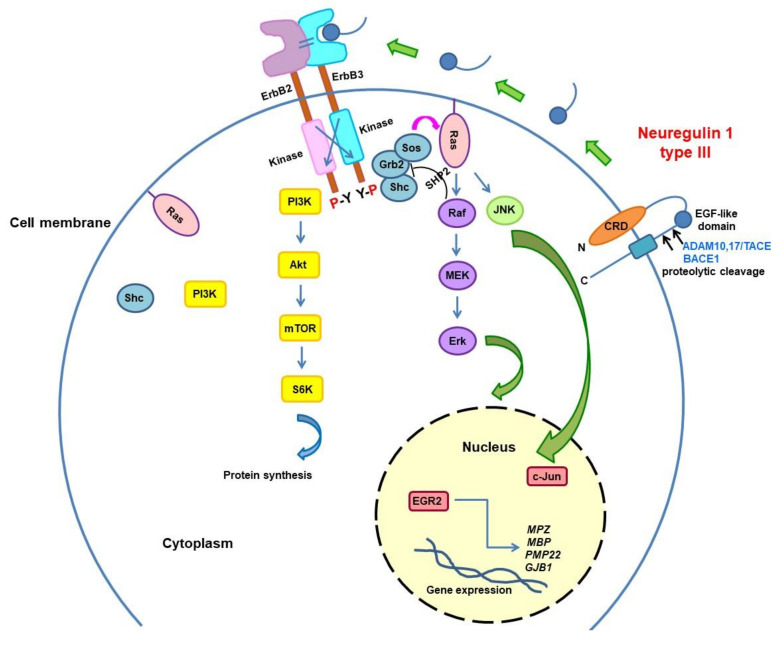
Figure 2.
Neuregulin1/ErbB signalling determines myelin sheath thickness. Neuregulin1 type III (Nrg1-III) has an epidermal growth factor-like (EGF-like) domain and contains a cysteine-rich domain (CRD) embedded in the lipid bilayer, which leaves the N-terminal side tethered to the membrane. Nrg1-III, produced by axons, is proteolytically cleaved by proteases of the ADAM family and BACE1. The BACE1 and TACE (also known as ADAM17) secretases are Nrg1-III regulators with opposite actions, the first by enhancing its activity and thus increasing myelin thickness, whereas the latter has an inhibitory effect. Nrg1-III provides the ligand for ErbB3 receptor leading to its heterodimerisation with ErbB2, activation of the tyrosine kinase domain, and phosphorylation of the cytoplasmic region of the ErbB partner. This event causes various adaptors/effectors’ (e.g., Ras-Shc, PI3K) recruitment and activation of multiple intracellular signalling pathways such as the PI3K–Akt and MAPK/Erk pathways in the Schwann cell.