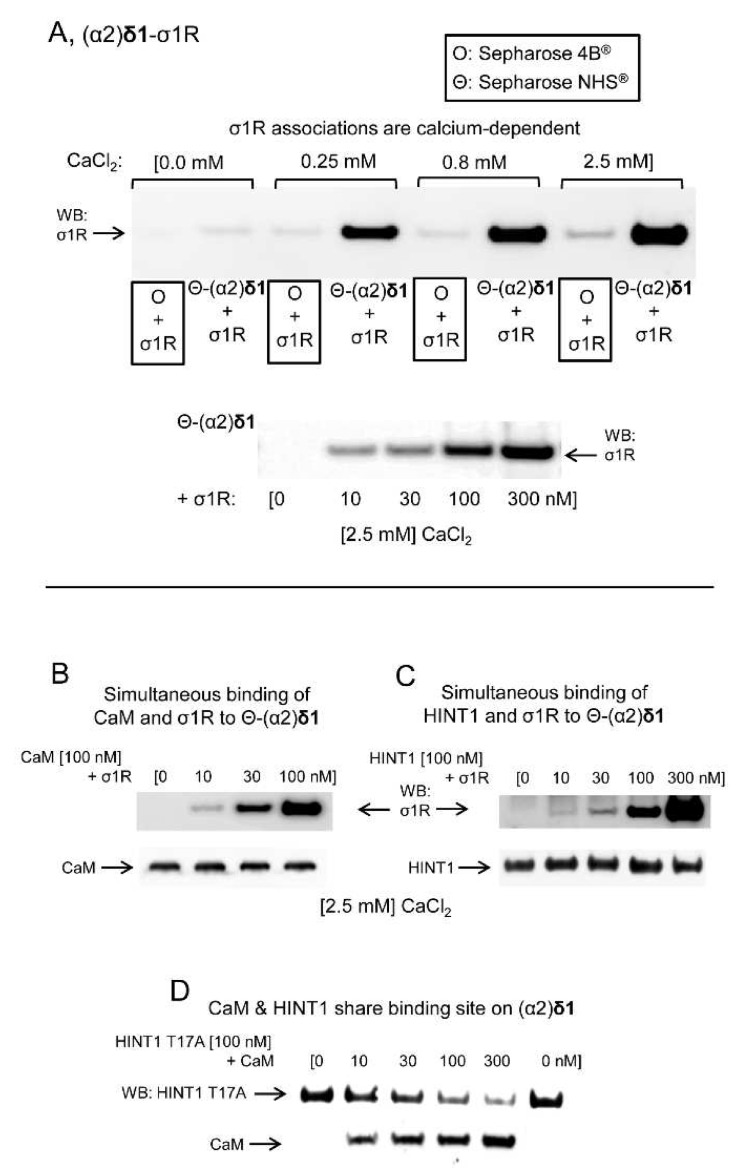
Figure 7.
Physical interactions of the (α2)δ1 peptide with σ1Rs, HINT1 proteins, and CaM. (A) Calcium-dependent binding of σ1Rs to (α2)δ1 peptides. Recombinant (α2)δ1 peptides covalently attached to NHS-activated Sepharose® were incubated with σ1Rs (100 nM) in the presence of increasing amounts of CaCl2. The pellets obtained were washed, solubilized in 2× Laemmli buffer containing β-mercaptoethanol, and resolved by SDS-PAGE. The presence of σ1R was analyzed in Western blots (WBs). The prey protein did not bind to NHS-Sepharose (O + σ1R, negative control). In another set of assays, (α2)δ1 peptides were incubated with increasing concentrations of σ1Rs in the presence of CaCl2 (2.5 mM). (B–D) Competition assays between σ1R, HINT1, and CaM for their binding to (α2)δ1 peptides. CaM (100 nM) was incubated with agarose-(α2)δ1 for 30 min at RT in 300 μL of 50 mM Tris-HCl, [pH 7.5], 0.2% CHAPS, CaCl2 (2.5 mM). After removal of the unbound CaM, increasing concentrations of σ1Rs were added. The (α2)δ1-bound proteins were detached, resolved by SDS-PAGE chromatography, and analyzed in Western blots (see Section 2). The assays were repeated at least twice, producing comparable results. This protocol was also used to assess competition between HINT1/σ1R and CaM/HINT1 in their binding to (α2)δ1 peptides. O and Θ represents plain agarose and NHS-Sepharose®, respectively.