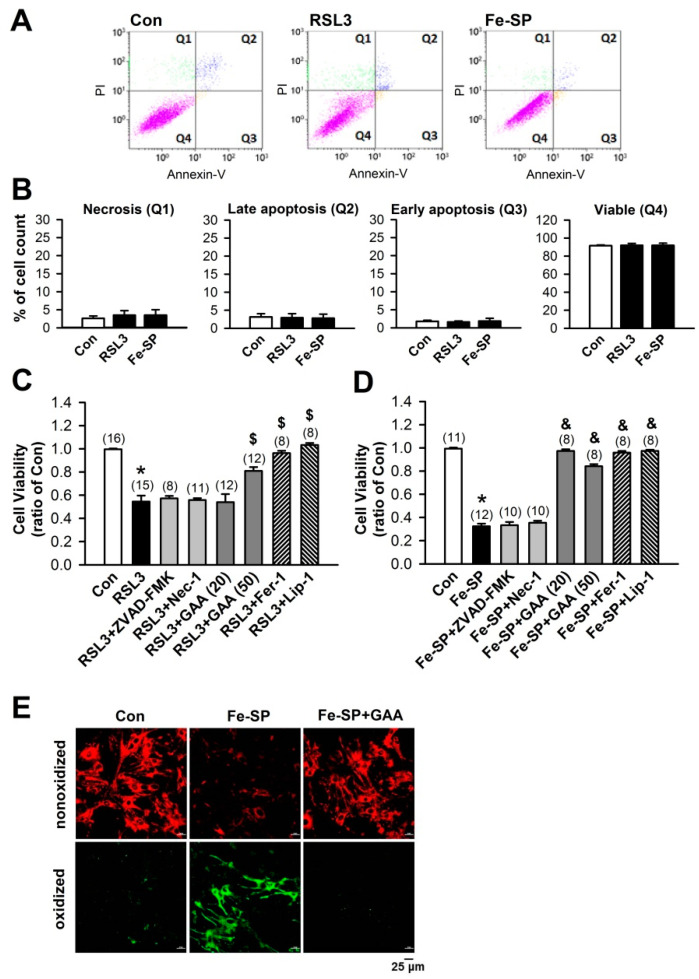
Figure 5.
GAA protects against ferroptotic cell death and inhibits lipid peroxidation in neonatal rat cardiomyocytes. (A) RSL3 and Fe-SP did not induce apoptosis and necrosis according to analysis by flow cytometry of Annexin V-FITC/PI double staining at 24 h. (B) Statistical analysis of necrosis (Q1), late apoptosis (Q2), early apoptosis (Q3), and viability (Q4) in neonatal rat cardiomyocytes (n = 4). (C) MTT results showing a significant decrease in cell viability 24 h postexposure to 0.5-μM RSL3, abolished by GAA (20–50 μM), Fer-1 (1 μM), and Lip-1 (2 μM), but not ZVAD-FMK (10 μM) or Nec-1 (10 μM). Values shown in parenthesis represent the number in each group. In each group, 2 to 3 samples were analyzed, and the same experiment was assessed by repeating cell culture four times or more. (D) MTT results showing a significant decrease in cell viability 24 h postexposure to 2-μM Fe-SP, abolished by GAA (50 μM), Fer-1 (1 μM), and Lip-1 (2 μM), but not ZVAD-FMK (10 μM) or Nec-1 (10 μM). Values shown in parenthesis represent the number in each group. In each group, 2 to 3 samples were analyzed, and the same experiment was assessed by repeating cell culture four times or more. (E) Confocal images of neonatal rat cardiomyocytes treated with Fe-SP (2 μM) for 2 h; a significant increase in levels of lipid peroxidation was observed (green), but this effect was abolished by 50-μM GAA. Red, nonoxidized form of C11 BODIPY; Green, oxidized form of C11 BODIPY. Scale bars: 25 μm. All data represent means ± SEM. * p < 0.05 versus the control group; $ p < 0.05 versus RSL3; & p < 0.05 versus Fe-SP. Con, control; Fer-1, ferrostatin-1; Lip-1, liproxstatin-1; RSL3, (1S,3R)-RSL3; Fe-SP, chlorido [N, N-disalicylidene-1,2-phenylenediamine] iron (III); GAA, gossypol acetic acid; Nec-1, necrostatin-1.