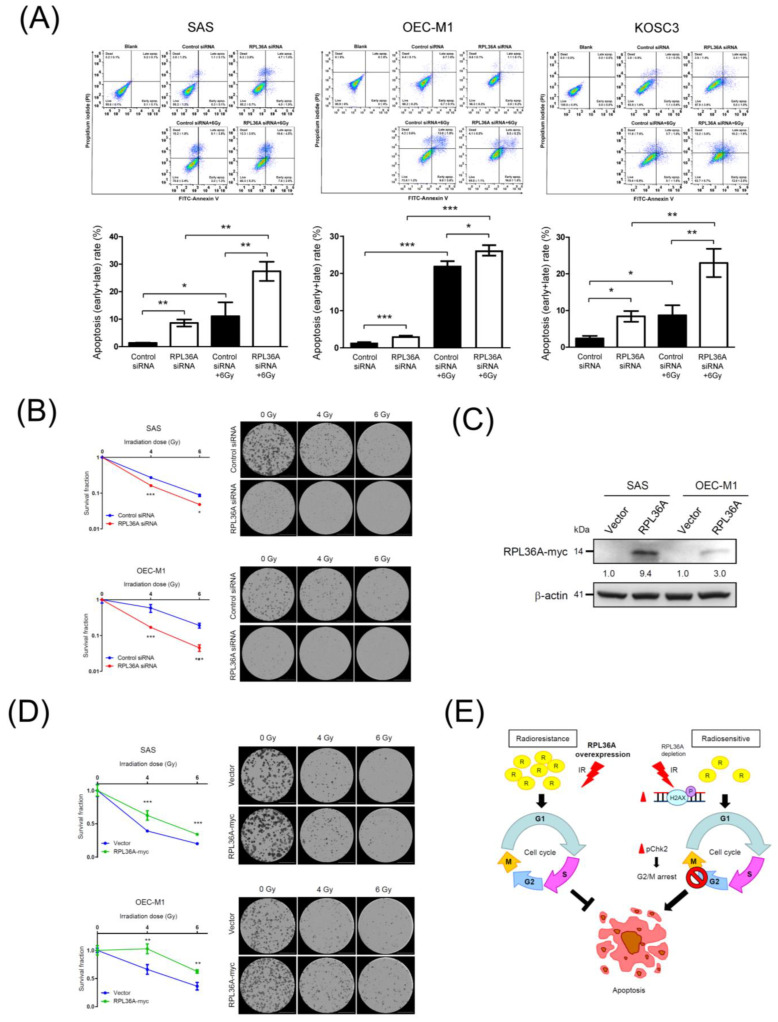
Figure 5.
RPL36A depletion augments apoptosis in response to irradiation: (A) SAS, OEC-M1, and KOSC3 cells were transfected with control siRNA and RPL36A-specific siRNA. After 6 Gy irradiation of SAS cells for 72 h, OEC-M1 cells for 96 h and KOSC3 cells for 48 h, apoptosis was determined by annexin V-FITC/PI staining followed by flow cytometry analysis. The following four populations were identified: live cells (annexin V-FITC and PI negative), early apoptotic cells (annexin V-FITC positive and PI negative), late apoptotic cells (annexin V-FITC positive and PI positive), and dead cells (annexin V-FITC negative and PI positive) (top). Quantification analysis of apoptosis (early + late) recorded in three independent experiments. Mean values of three independent experiments ± SD are shown. The p-values were calculated by using unpaired Student’s t-test. * p < 0.05 and ** p < 0.01 (bottom). Clonogenic survival assays performed with RPL36A-knockdown (B) and RPL36A-expressing (D) cells after exposure to different doses of irradiation (0, 4, and 6 Gy). (C) Western blotting analysis was used to determine RPL36A expression levels in RPL36A-expressing SAS and OEC-M1 cells. β-Actin was used as an internal control. The number of colonies (>50 cells/colony) was counted and analyzed by a Lionheart FX automated microscope. Data are presented as the mean values obtained from three independent experiments. Error bars indicate SD. * p < 0.05, ** p < 0.01, and *** p < 0.001. (E) Hypothetical schematic of a regulatory role of RPL36A in developing radioresistance in OSCC. In OSCC cells, elevated RPL36A levels triggered a radioresistance phenotype. Furthermore, under a condition of RPL36A repression, the decreased RPL36A level sensitized cells to DNA damage and induced cell cycle G2/M arrest followed by augmenting irradiation-induced apoptosis pathway, as a consequence of increased OSCC radiosensitivity.