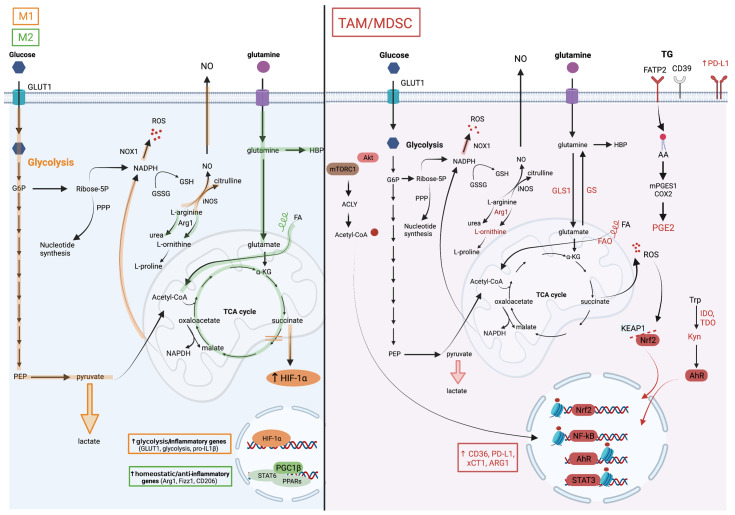
Figure 2.
Metabolic pathways associated with TAM and MDSC pro-tumoral and myelosuppressive functions. The M1/M2 paradigm established from an in vitro macrophage culture system distinguishes two extreme macrophage metabolic phenotypes. (On the left), the metabolic pathways in orange are upregulated in M1 macrophages and those in green in M2 macrophages. M1 macrophages are highly glycolytic which is a consequence of HIF-1a stabilization in response to succinate accumulation due to breaks in the TCA cycle. Besides inducing the expression of glycolytic enzymes, HIF-1a also drives the production of pro-inflammatory factors such as IL-1b. In contrast, M2 macrophages favor glutamine consumption, upregulate the hexosamine biosynthetic pathway (HBP), and rely on fatty acid oxidation (FAO) for their energetic needs. STAT6 downstream of IL-4 signaling and PPARg with its co-activator PGC1b are key for their differentiation. In contrast to this simplified system, the TME complexity results in a marked myeloid cell heterogeneity with M1/M2 mixed profiles and divergent metabolic characteristics. (On the right), the cellular metabolic pathways upregulated in TAMs and MDSCs are illustrated. These cells are highly glycolytic but are dependent on glutamine and lipid consumption for their pro-tumoral functions. Despite their heightened aerobic glycolysis, they upregulate M2-like genes through the accumulation of acetyl coA, which is downstream of the AKT/mTOR-dependent activation of ATP Citrate Lyase (ACLY), and histone acetylation. Histone lactylation, which was reported to occur in M1 macrophages at later stages of activation and proposed as a mechanism to terminate the inflammatory response, might regulate TAM functions, but this remains to be tested. The heightened mitochondrial respiration in tumor-associated myeloid cells leads to the elevated production of reactive oxygen species (ROS). To withhold oxidative stress, they activate the transcription factor NRF2, which induces the expression of anti-oxidative genes and of the cystine transporter xCT1, among others. Myeloid cells in the TME upregulate triglycerides (TG) uptake, for instance through fatty acid transport protein (FATP)2, as reported in granulocytic 18 MDSCs, lipid accumulation in vesicles, lipolysis and FAO. Consequently, they also produce inflammatory and immunosuppressive lipid mediators such as the prostaglandin PGE2. Furthermore, they metabolize arginine and tryptophan into metabolites that favor tumor growth, including L-ornithine and L-kynurenine (Kyn). The latter is a ligand of aryl hydrocarbon receptor (AHR), which promotes myelosuppressive functions in the TME via transcriptional activity.