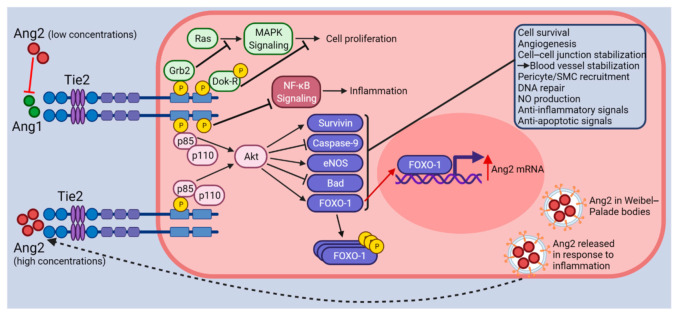
Figure 1.
Illustration of Ang-Tie2 signaling. The Tie2 receptor undergoes autophosphorylation upon binding of Ang1. Subsequently, PI3K (p85/p110) and Akt signaling are activated, which in turn promote survival and anti-apoptotic signals through the upregulation of Survivin and eNOS and the inhibition of Caspase-9 and Bad. FOXO-1 transcription factors are involved in protein synthesis. Following Tie2 activation (effects shown with black arrows and inhibitor lines), FOXO-1 is phosphorylated and inactivated, which promotes EC quiescence, survival, and vascular stabilization. Other proteins associated with phosphorylated Tie2 such as Grb2 and Dok-R inhibit cell proliferation. ABIN-2 (not shown) associates with phosphorylated Tie2 and is thought to prevent NF-κB signaling activation through the inhibition of the IKK complex. Altogether, Tie2 activation (by Ang1 or high concentrations of Ang2) leads to vessel stabilization through EC survival, quiescence, and the stabilization of cell–cell junctions. When Ang2 binds to Tie2 at lower concentrations (effects shown with red arrows and inhibitor lines), it acts as a Tie2 antagonist preventing Ang1 from binding to Tie2. Acting as an antagonist to Tie2 signaling, Ang2 binding causes vessel destabilization, pericyte/SMC drop-off, cell migration, inflammation, and vascular leakage. Under these conditions, the FOXO-1 transcription factors are activated and promote the transcription of Ang2 mRNA, vascular destabilization, and apoptosis. Finally, the Ang2 protein produced is stored in Weibel–Palade bodies, ready for release into the ECM upon the detection of inflammatory signals. Figure created with BioRender (accessed on 15 November 2021).