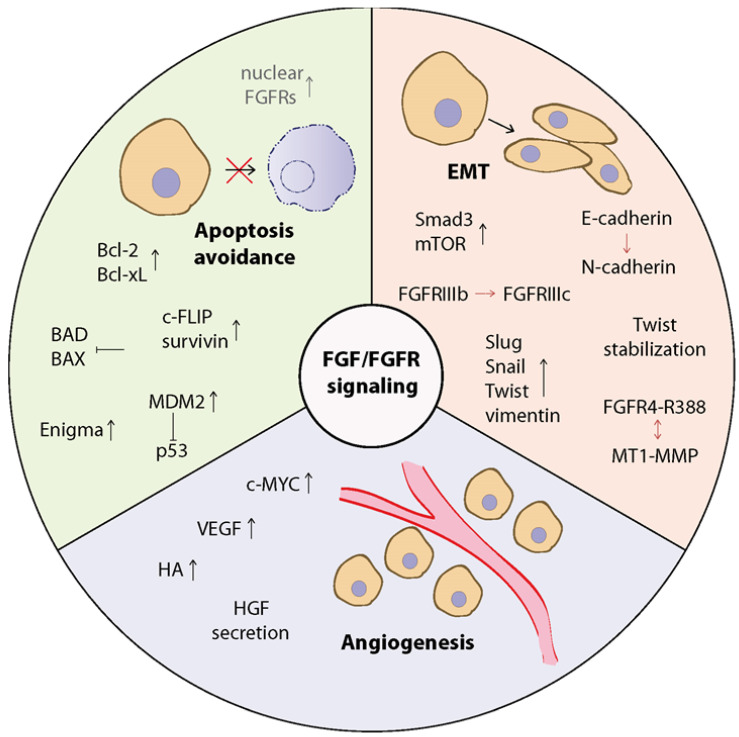
Figure 3.
Involvement of FGF/FGFRs in cellular processes during the development of drug resistance. The active FGF/FGFR complex leads to avoidance of apoptosis through increased expression of apoptosis inhibitors (Bcl-2, Bcl-xL), inhibition of its activators (BAD, BAX) or activation and stabilization of MDM2, with consequent increased ubiquitination and degradation of p53. Additionally, p53 degradation can be enhanced by the formation of a ternary complex of p53, MDM2, and Enigma protein. Another mechanism of action is indirect inhibition of caspase 3/7 and caspase 8 by increasing the expression of survivin or c–FLIP, respectively. It is also likely that increased nuclear localization of FGFRs may be associated with enhanced survival of cancer cells and development of drug resistance. FGF/FGFR (especially involving FGF2) also promotes angiogenesis through increased expression of VEGF, HA, and c-MYC, and enhanced secretion of HGF. EMT, in turn, is a consequence of activation of the MAPK signaling pathway leading to stabilization of Twist, switching of FGFR isoforms from IIIb to IIIc, switching of cadherins (from E-cadherin to N-cadherin), upregulation of mesenchymal markers (such as Twist and vimentin) and transcription factors (Slug and Snail), and activation of downstream signaling proteins (such as Smad3 and mTOR). Furthermore, the FGFR4-R388 mutant can interact with matrix metalloproteinases (e.g., MT1-MMP), proteins involved in tumor invasion.