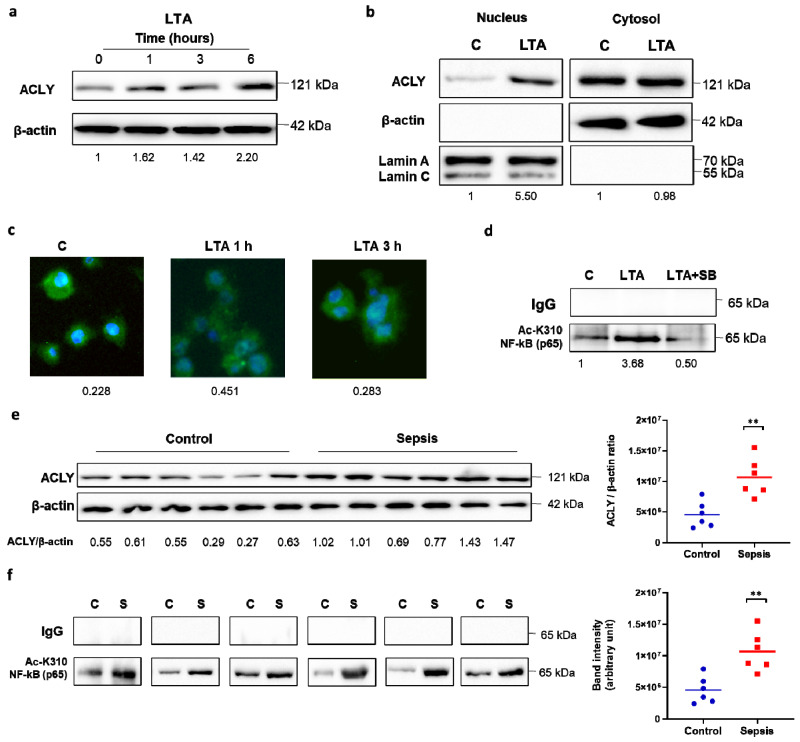
Figure 4.
LTA and sepsis in the early hyperinflammatory phase triggered ACLY-mediated NF-κB acetylation. Human PBMC-derived macrophages were triggered by LTA, and (a) western blot experiments were performed to evaluate ACLY protein. (b) Nuclei were isolated by cell fractionation. In nuclear and cytoplasm fractions, ACLY, β-actin, and lamin proteins were detected by specific antibodies. (c) Time course of ACLY nuclear translocation by immunocytochemistry (ICC) using anti-ACLY and DAPI nuclear staining in iBMDM cells treated with LTA. (d) In human PBMC-derived macrophages triggered by LTA, with or without SB, NF-κB (p65) was immunoprecipitated with a specific antibody and then analyzed by western blotting with an anti-NF-κB p65 (acetyl K310) antibody. (e,f) Macrophages differentiated from PBMCs of patients with sepsis in the early hyperinflammatory phase and age-matched healthy controls were used to quantify ACLY protein by western blot experiments (e) and to immunoprecipitate NF-κB (p65) with a specific antibody and then to analyze by western blotting with an anti-NF-κB p65 (acetyl K310) antibody. (f) Western blotting data presented are representative of at least 3 independent experiments. In (a,b,e), protein levels are quantified against β-actin. In (a,b,d), the mean of the protein values was normalized versus the mean of the proteins in untreated cells (C), and the results are reported under each image. In (c), quantitation of ACLY in ICC experiments was normalized to the DAPI nuclear stain. In (e,f), statistical significance of the differences between the ACLY amount in the controls and sepsis samples evaluated by using a Mann–Whitney U test (** p < 0.01) is depicted in dot plots (right panels).