Figure 3.
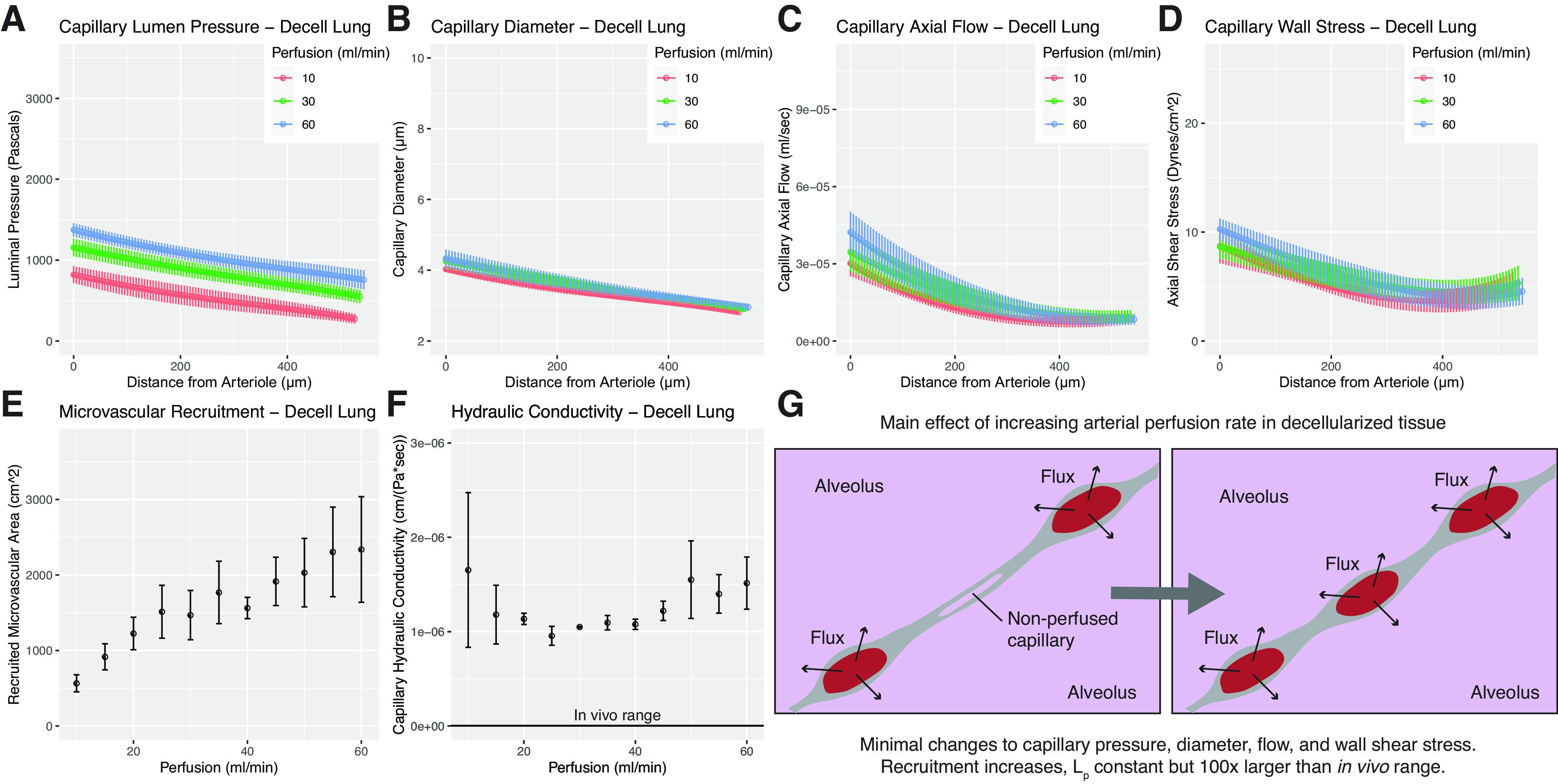
Model solutions for decellularized tissues. A–F: represent model outputs for decellularized lungs. A: microvascular pressure in decellularized lung. Increasing arterial perfusion is found to only marginally increase intramural pressures. B: fluid readily passes into the alveolus in decellularized tissue, and our model predicts that capillary diameter in decellularized tissue is independent of perfusion rate in the range tested. C: axial flow in decellularized lung is found to be nonlinear and substantially lower than in native lung. D: microvascular wall shear stress in decellularized lung is predicted to be higher than that of native at the arteriolar inlet, while approaching more comparable values at the venule. E: recruitment is found to increase steadily with increasing perfusion rate. F: hydraulic conductivity in decellularized lung is found to be two orders of magnitude larger than in native tissue. In vivo range shown for reference. G: visual schematic showing changes in decellularized lung microvascular steady state due to increased perfusion. Benchtop experiments yielding input values for modeling were performed using n = 3 tissues. Model solutions for each experimental run were then averaged to yield mean values, shown here. Error bars in all graphs represent ± standard error of the mean across experiments.
