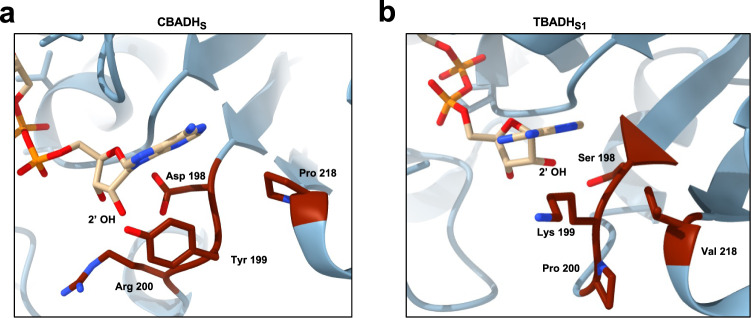
Fig. 3. Crystal structures of CBADHS and TBADHS1 reveal mechanism of cofactor selectivity reversal.
a Cofactor binding site of CBADHS with NAD+ bound. The substitutions identified in CBADHS allow for the binding of NAD+ but would prevent the binding of NADP+ by steric impediments and electrostatic repulsion with the side chains of D198 and Y199. Additionally, the stacked-ring interaction of the adenine moiety with Y218 observed in CBADHWT cannot be established due to the Y218P substitution, possibly enabling a more flexible binding of the cofactor. b Cofactor binding site of TBADHS1, with NAD+ placed at the same position as NADP+ in the structure of TBADHWT. P200−P201 is modelled as a cis peptide bond. While TBADHS1 can accommodate NAD+ in its cofactor binding pocket, the binding of NADP+ would be prevented by steric impediments caused by the side chains of S198 and K199. As in CBADHS, the substitution of Y218 prevents the formation of a stacked-ring interaction with the adenine ring of the cofactor, possibly enabling a more flexible binding of NAD+.