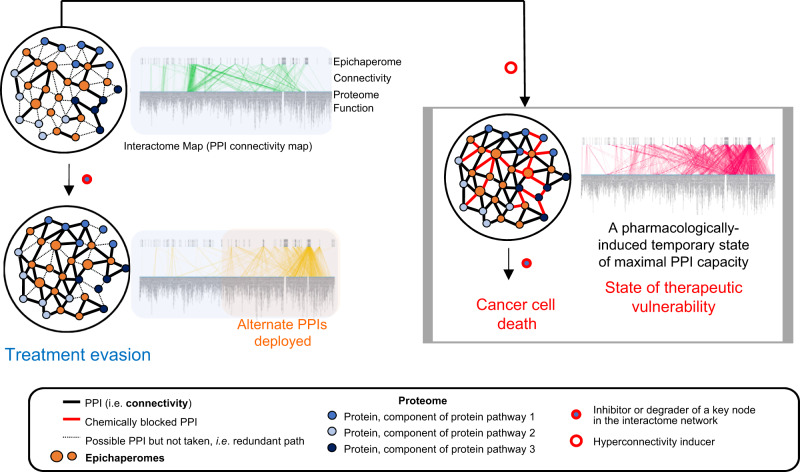
Fig. 10. Summary of findings.
Interactome network plasticity in cancer, which arises from highly redundant signalling pathways, poses a real challenge to therapy and accounts for treatment resistance. Herein, we leverage discoveries in the biology of cellular stress linking cellular vulnerability to hyperconnectivity in PPI networks to propose a method for pharmacologically rewiring PPI networks at proteome-wide level for therapeutic vulnerability. By employing this approach, we provide proof-of-principle in cancer that by pharmacologically controlling interactome connectivity through epichaperomes, we anticipate the trajectory of PPI changes and co-opt dynamic cellular PPI networks to in situ engineer a state of hyperconnectivity. By forcing PPI networks into a hyperconnected state, we create therapeutic sensitivity, effectively making controllability into a powerful treatment strategy. This means existing treatments may be more effective than previously observed if controllability measures are exercised in tandem with drug treatment whereby cancerous cells are forced into a state of interactome hyperconnectivity. Several inhibitors/degraders of PPI nodes are already in clinical use or in development (ex. inhibitors or degraders such as PROTACS and others that target nodes in signalling networks), making this approach both timely and impactful. This treatment paradigm is not a combination method per se, because the hyperconnectivity inducer is used once to prime the tumour, followed by current therapy. It also differs from synthetic lethality where simultaneous perturbation of two or more genes is required for cellular death.