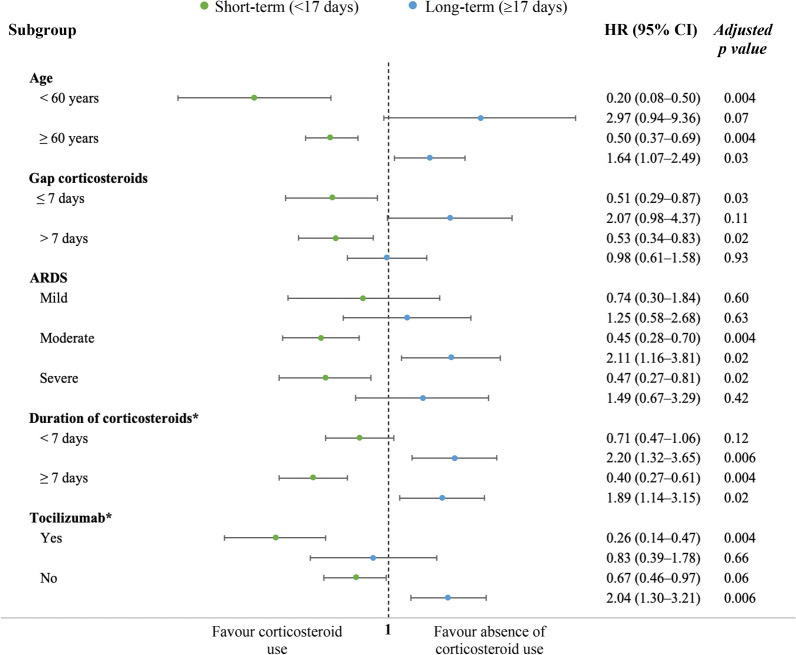
Fig. 4.
Subgroup sensitivity analysis. Propensity score matching was performed for each study subgroup as in the primary analysis to evaluate the estimated effect of corticosteroids on ICU mortality over time with cause-specific hazard model. Post hoc analysis was made for subgroups of duration of corticosteroid treatment and Tocilizumab. GAP corticosteroids mean the time (in days) since onset of symptoms to corticosteroid initiation. Models were adjusted for gender, age, body mass index, hospital GAP, ICU GAP, diagnosis GAP, shock, ACE inhibitors, ARBs, Comorbidity, asthma, COPD, chronic kidney disease, haematological disease, diabetes mellitus, neuromuscular disease, autoimmune disease, ischaemic heart disease, hypertension, immunosuppression, dyslipidaemia, hypothyroidism, APACHE II, SOFA, pulmonary infiltrates, lactate dehydrogenase, white blood cells count, creatinine, urea, C-reactive protein, procalcitonin, Lactate, d-dimer, antibiotics, oseltamivir, lopinavir plus ritonavir, remdesivir, interferon, hydroxychloroquine, Tocilizumab, bacterial co-infection, ARDS severity, fractional of inspired oxygen (FiO2), positive end-expiratory pressure, tidal volume, partial pressure of carbon dioxide, pH, RIFLE criteria, myocardial dysfunction and corticosteroid treatment (short and long-term). ICU intensive care unit, ACE angiotensin-converting enzyme, ARBs angiotensin receptor blockers, COPD chronic obstructive pulmonary disease, APACHE acute physiology and chronic health evaluation, SOFA sequential organ failure assessment, ARDS acute respiratory distress syndrome, CRP C-reactive protein