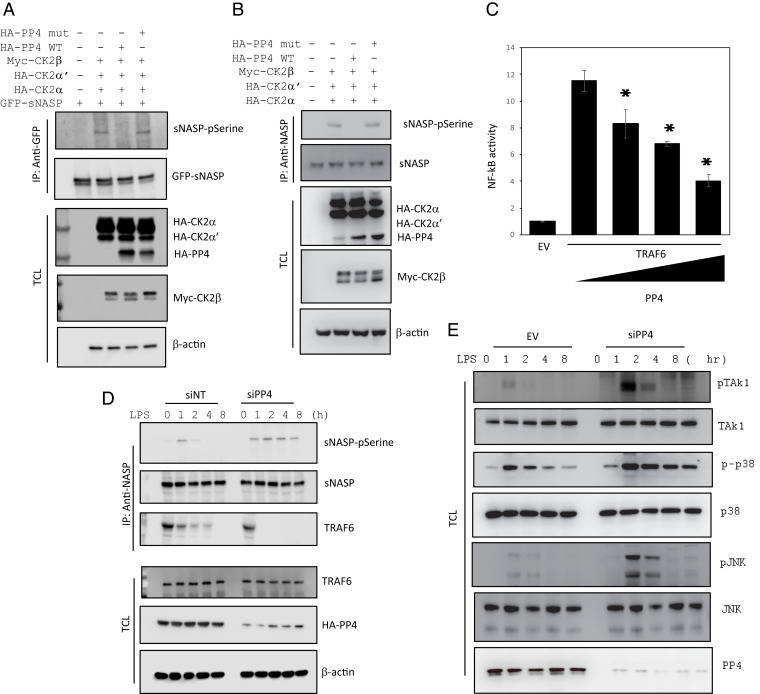
Fig. 1.
PP4 dephosphorylates sNASP and inhibits LPS-induced NF-κB activation. (A) IP of sNASP (with anti-GFP) from HEK293 cells transfected with GFP-tagged sNASP in the presence (+) or absence (−) of Myc-tagged CK2β, HA-tagged CK2 catalytic subunits (CK2α and CK2α’), HA-tagged WT PP4 (HA-PP4), or mutant (mut), assessed by IB with antibodies against phosphorylated serine (pSerine) or anti-GFP after IP with anti-GFP. TCL was IB with anti-HA, anti-Myc, or anti–β-actin. (B) Cell lysates from THP-1 cells transduced with indicated CK2 subunits, HA-PP4 WT or mut was immunoprecipitated using anti-sNASP and immunoblotting conducted as in A. (C) Luciferase activity in HEK293 cells transfected with a luciferase reporter vector driven by an NF-κB-responsive promoter, plus EV or vector encoding TRAF6 and increasing concentrations of a vector encoding PP4. Results were standardized to EV (set as 1). Data are the mean ± SE for each group. *P < 0.05 (by Student’s t test). (D) THP-1 cells were transfected with siRNA negative control (siNT) or siPP4, followed by IB with antibodies against phosphorylated serine (pSerine), TRAF6, or sNASP after IP with anti-sNASP. TCL IB was done with anti-TRAF6, anti-HA, or anti–β-actin. (E) IB of indicated antibodies in LPS-stimulated THP-1 cells transduced with siNT or siPP4. Data represent a minimum of three independent experiments.