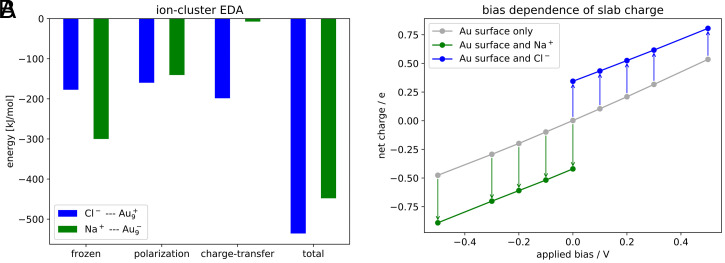
Fig. 4.
Results of electronic structure calculations. (A) Comparison of the different contributions to the interaction energy of the Na+ (green) and Cl− (blue) ion with a negatively and positively charged Au9 cluster model, respectively, as calculated with ωB97X-V/def2-TZVPD. While the polarization term is of equal magnitude, charge transfer is completely absent in case of Na+, whereas it contributes to the interaction energy for the Cl− case. (B) The net charge of a grand-canonical periodic slab model with varying bias potential referenced against the computed PZC. The green (blue) data points show the corresponding decrease (increase) of the net charge upon Na+ (Cl−) binding. The change of this polarization effect is negligible in the experimental bias range (−0.15 to 0.2 V) as indicated by the constant length of the colored arrows.