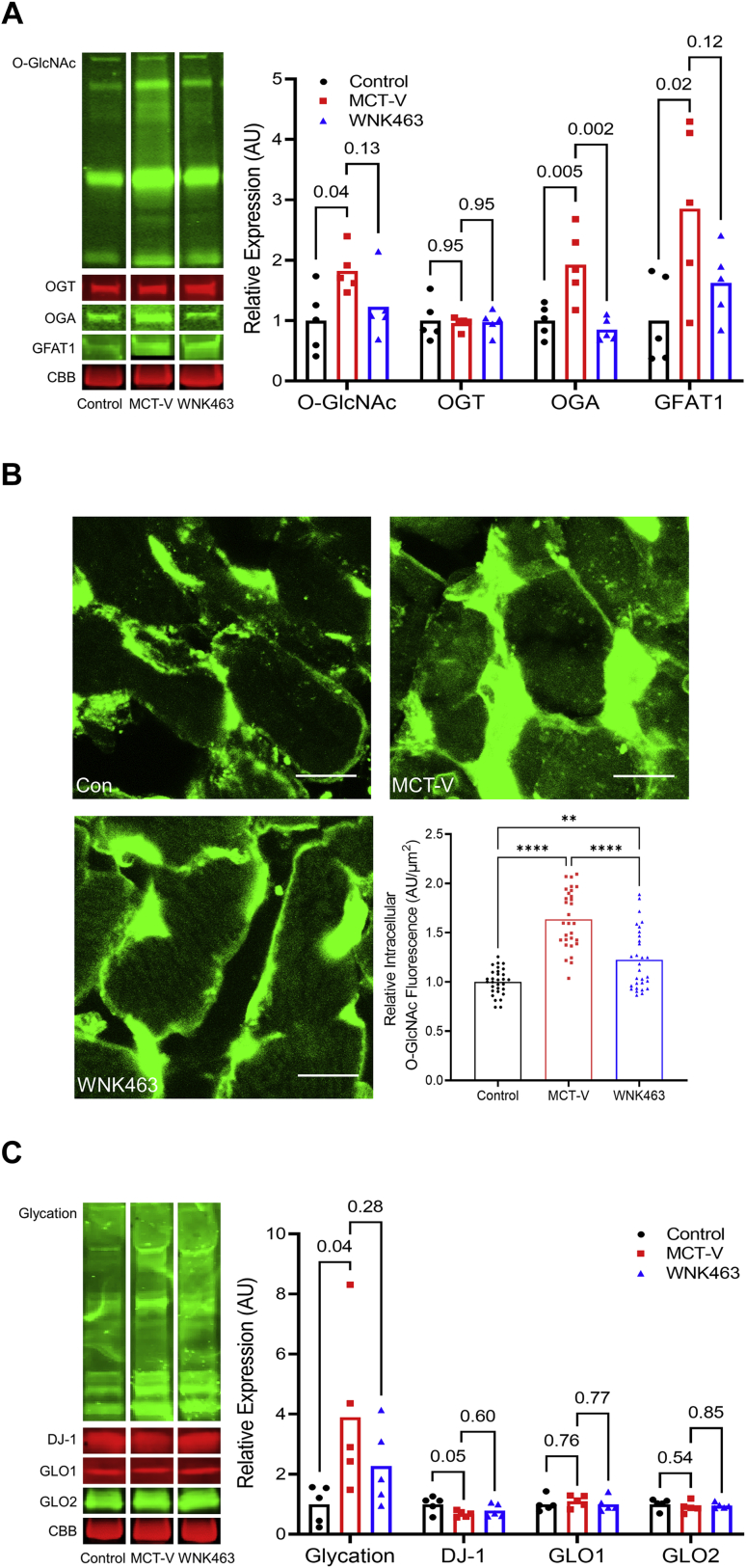
Figure 2.
Protein O-Glcnacylation and Glycation Are Blunted by WNK463
(A) Representative Western blots and quantification of protein abundance in RV extracts from control, MCT-V, and WNK463 rats demonstrate WNK463 trends toward normalizing protein O-GlcNAcylation (control rats: 1.0 ± 0.5; MCT-V: 1.8 ± 0.4; WNK463: 1.2 ± 0.5 expression relative to control rats; n = 5 RVs per group for all proteins assessed), O-GlcNAcase (OGA) (control rats: 1.0 ± 0.3; MCT-V: 1.9 ± 0.6; WNK463: 0.9 ± 0.2), and glutamine-fructose-6-phosphate transaminase 1 (GFAT) (control rats: 1.0 ± 0.7; MCT-V: 2.9 ± 1.4; WNK463: 1.6 ± 0.6) expression and did not change O-linked β-N-acetylglucosamine transferase (OGT) abundance (control rats: 1.0 ± 0.3; MCT-V: 1.0 ± 0.1; WNK463: 1.0 ± 0.2). Western blot results were normalized to the myosin heavy chain band in the CBB stained post-transfer gel. Values are expression relative to control rats. (B) Representative confocal micrographs of RV free wall sections stained with succinylated wheat germ agglutinin (WGA) show increased intracellular O-GlcNAcylation signal in MCT-V, which is reduced by WNK463. Scale bar 10 μm. n = 3 RVs per group, 10 areas assessed per RV. (C) Representative Western blots and quantification of protein glycation, DJ-1, glyoxalase 1 (GLO1), and GLO2 reveal WNK463 nonsignificantly reduces total protein glycation (control rats: 1.0 ± 0.6; MCT-V: 3.9 ± 2.7; WNK463: 2.3 ± 1.3) without altering DJ-1, GLO1, and GLO2 abundance in the RV. One-way ANOVA with Dunnett post hoc analysis was completed in A and C. ∗∗P < 0.01 and ∗∗∗∗P < 0.0001 by Brown-Forsythe and Welch ANOVA with Dunnett multiple comparison test in B. Abbreviations as in Figure 1.