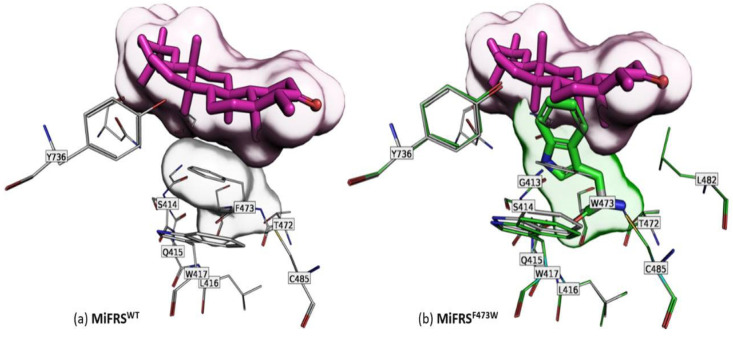
Figure 4.
Secondary alignment (or side-by-side representation) of wild protein (MiFRS) (gray-stick carbons) with the MiFRSPhe473Trp mutant (green-stick carbons). The surface highlights the volume adopted by the friedelin molecule in the catalytic cavity. The green stick indicates the mutated residue. (a) Favorable interaction between the wild-type Phe473 residue and substrate. (b) Steric bulk of Trp473 residue at the active site of MiFRSPhe473Trp. Interaction between Trp417 and Trp473 residues is favorable, which blocks the substrate entrance inside the pocket.