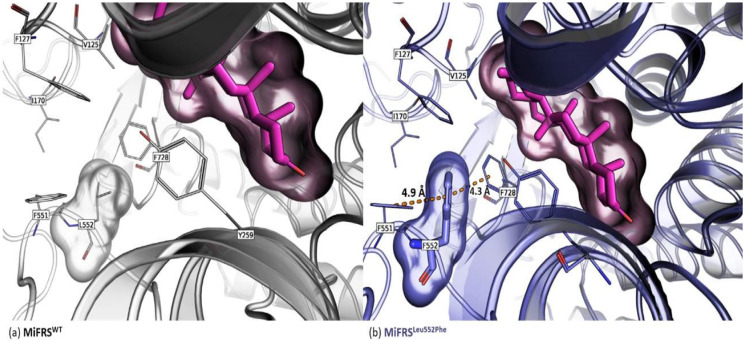
Figure 5.
Secondary alignment (or side-by-side representation) of wild protein (MiFRS) (gray-lines carbons) with the MiFRSLeu552Phe mutant (blue-lines carbons). The surface highlights the volume adopted by the friedelin molecule in the catalytic cavity. The blue stick residue indicates the mutated residue. (a) The mutation approached the Phe728 (not shown) residue of catalytic site, with a π-electron type interaction that stabilizes the C-ring, decreasing the enzyme specificity. (b) Another possibility of rotamer is impaired due to the collapse of other main or side chain residues.