Abstract
Alpha-synucleinopathies comprise progressive neurodegenerative diseases, including Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA). They all exhibit the same pathological hallmark, which is the formation of α-synuclein positive deposits in neuronal or glial cells. The aggregation of α-synuclein in the cell body of neurons, giving rise to the so-called Lewy bodies (LBs), is the major characteristic for PD and DLB, whereas the accumulation of α-synuclein in oligodendroglial cells, so-called glial cytoplasmic inclusions (GCIs), is the hallmark for MSA. The mechanisms involved in the intracytoplasmic inclusion formation in neuronal and oligodendroglial cells are not fully understood to date. A possible mechanism could be an impaired autophagic machinery that cannot cope with the high intracellular amount of α-synuclein. In fact, different studies showed that reduced autophagy is involved in α-synuclein aggregation. Furthermore, altered levels of different autophagy markers were reported in PD, DLB, and MSA brains. To date, the trigger point in disease initiation is not entirely clear; that is, whether autophagy dysfunction alone suffices to increase α-synuclein or whether α-synuclein is the pathogenic driver. In the current review, we discuss the involvement of defective autophagy machinery in the formation of α-synuclein aggregates, propagation of α-synuclein, and the resulting neurodegenerative processes in α-synucleinopathies.
Keywords: alpha-synuclein, Parkinson’s disease, multiple system atrophy, autophagy, neurons, oligodendroglia
1. Introduction
The term α-synucleinopathies (ASPs) was introduced to categorize a group of disorders that feature pathological α-synuclein (α-syn) accumulations in neuronal and/or glial cells throughout the central nervous system (CNS). Inclusions of α-syn and neuroinflammatory response have also been reported to occur in the peripheral nervous system (PNS) [1,2,3]. ASPs cover Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA) and are adult-onset, progressive, neurodegenerative diseases [4]. They differ in the occurrence of α-syn-positive deposits; in PD and DLB, the abnormal aggregates appear preferentially in neurons—termed Lewy bodies (LBs) and Lewy neurites [5,6], whereas MSA displays the α-syn-positive aggregates predominantly in oligodendroglial cells, which are called glial cytoplasmic inclusions (GCIs) [7,8]. The accumulation is thought to be caused by a diminished degradation of the aberrant form of α-syn in neurons and glia, leading to increased levels of toxic α-syn species, inducing neurodegenerative events [9,10,11]. There is accumulating evidence that neurodegeneration can also be initiated and caused by inflammatory events. Microglial and astroglial cells can be activated by various α-syn forms, among others (e.g., brain injury and infections), leading to an immunological reaction in the brain. If the insult lasts too long, as in neurodegenerative diseases, a kind of feedback loop evolves and microglia and astroglia can become over-activated, so-called reactive micro- and astrogliosis, and lead to neuronal loss as well [12,13,14].
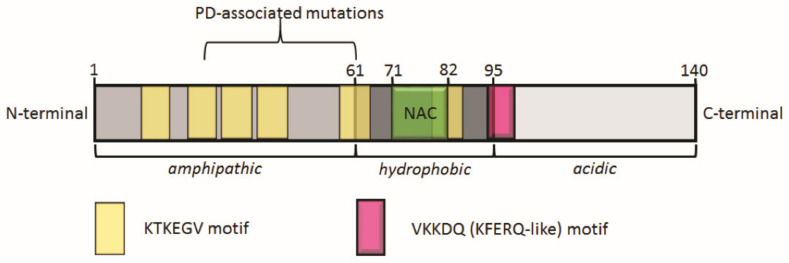
α-syn is a neuronal protein and is localized predominantly in presynaptic terminals of the striatum, hippocampus, thalamus, cerebellum, and neocortex, respectively [15,16]. Its function in a healthy brain is not fully elucidated to date, but includes regulating synaptic vesicle maintenance and recycling, as well as a role in synaptic plasticity [17,18,19]. The protein is part of a distinctive family of genes that includes SNCA—α-syn, SNCB—β-syn, and SCNG—γ-syn [20,21]. α-syn contains 140 amino acids and comprises three distinctive regions: (1) residues 1–60 form an amphipathic N-terminus including repeats of the conserved KTKEGV motif, (2) residues 61–95 assemble to form a non-amyloid β-component (NAC) region—a hydrophobic area, and (3) residues 96–140 build an acidic C-terminus [22]. The NAC region (residues 71–82) and a short motif in the N-terminal region (residues 36–42) in the center of the α-syn gene were shown to be involved in its aggregation [23,24]. The structure of α-syn includes a motif for binding of the heat shock cognate 70 (Hsc70) chaperone, VKKDQ (KFERQ-like, residues 95–99), leading to the degradation of α-syn via the chaperone-mediated autophagy (CMA) pathway (Figure 1) [25,26,27].
Figure 1.
Schematic representation of full-length α-synuclein structure (140 amino acids). α-synuclein consists of an amphipathic region from amino acid 1 to 61 with several repetitions of the conserved KTKEGV motif. This region is involved in membrane binding. The hydrophobic part of α-synuclein extends from amino acid 62 to 95 and comprises the non-amyloid β-component (NAC) region, which is important for promoting aggregation. From amino acids 96 to 140, α-synuclein features an acidic region, which is involved in ligand binding. Moreover, α-synuclein holds a VKKDQ (KFERQ-like) motif that is the binding site for heat shock cognate 70 (Hsc70) inducing degradation via chaperone-mediated autophagy (CMA) [25].
Pathways contributing to the degradation of wild type or soluble α-syn in healthy cells include the autophagic machinery and the proteasomal degradation pathway [28,29], whereby the autophagy pathway was recognized as the more common degradation process of misfolded α-syn [30,31]. Regarding the pathogenesis of ASPs, a contribution of a disrupted autophagy-lysosomal pathway (ALP) has been demonstrated to play a major role in the formation of α-syn-positive aggregations [32,33,34,35]. The autophagic system in these neurodegenerative disorders may reach a saturation point in which protein aggregates are not degraded anymore. In a vicious cycle, protein aggregates may be also responsible for the sequestration of autophagy proteins that can no longer contribute to the formation of the autophagic machinery [11].
The ALP in the cell is an adaptive machinery responsible for the degradation of defective and redundant cellular components, as well as long-lived proteins, thereby providing the cell nutrients for indispensable cellular functions, including fasting and other forms of stress and, furthermore, cytoprotection. Autophagy is a cellular process that needs stringent control to secure the correct response to different cellular stimuli. The term autophagy comprises three different forms: (1) microautophagy, (2) macroautophagy, and (3) CMA. Microautophagy will not be further discussed in the current review, as no data exist on microautophagy involvement in the degradation of α-syn. In contrast, the other autophagy forms, i.e., macroautophagy and CMA, seem to be relevant for the α-syn degradation process [36].
Macroautophagy (commonly called autophagy) begins with the generation of autophagosomes, which are double-lipid membrane structures involved in degrading cytosolic constituents, such as organelles and proteins. In a next step, the autophagosomes fuse with lysosomes, yielding autophagolysosomes, in which the proteins or organelles are subsequently degraded by hydrolases. The developing free amino acids are then again available to the cell for the building of new cellular constituents. Numerous proteins take part in the formation of the autophagosome, including the 15 so-called autophagy-related proteins (ATGs, AuTophaGy), first described in yeast [37]. Among others, the autophagy-related protein microtubule-associated protein 1 light chain 3B (LC3B) and Beclin-1 are significant players in autophagosome generation and are common markers used in measuring macroautophagy activity. The lipidated form of LC3, LC3-II, is found specifically on the membrane of autophagosomes [38]. Furthermore, the ubiquitin-binding protein, p62, a receptor for recognizing poly-ubiquitinated proteins and LC3, act together and escort these protein aggregates to the autophagosomes [39,40]. Macroautophagy is controlled by different upstream proteins, including UNC-51-like kinases (ULK1, ULK2), vacuolar protein sorting-associated protein 34 (VPS34), and autophagy/beclin1 regulator 1 (AMBRA1). Macroautophagy commences by the activation or modification of these upstream proteins, thereby blocking the mammalian target of rapamycin (mTOR) [41]. For a detailed report on macroautophagy and its key players, see the reviews from Dikic I. and Elazar Z. and Yang Z. and Klionsky D.J. [42,43].
CMA is a highly selective autophagy pathway and, compared with micro- and macroautophagy, no vesicle formation occurs during the degradation process. Importantly, for the recognition of the substrate by a group of chaperones (including Hsc70 chaperone), a CMA targeting motif is necessary. Only proteins containing this motif, a sequence biochemically related to the KFERQ pentapeptide, will be recognized and escorted directly to the lysosome [44]. At the lysosomal membrane, interaction of the proteins with the cytosolic end of the lysosome-associated membrane protein type 2A (Lamp-2A) will occur. Lamp-2A is a transmembrane protein that helps to translocate the substrate through the lysosomal membrane, where a rapid degradation process by hydrolytic enzymes takes place [45].
As the accumulation of α-syn is one of the fundamental events in the initiation and advancement of PD, DLB, and MSA, unravelling the cause behind the aggregation process is highly desired to understand these neurodegenerative diseases and to generate new medication that will halt the worsening of ASPs. In the current review, we will highlight recent findings that claim an involvement of autophagy, including macroautophagy and CMA, in disease initiation and progression of ASPs. Thereby, we will elaborate on differences and similarities regarding autophagy impairment in neuronal ASPs, including PD and DLB, compared with oligodendroglial ASPs, namely MSA. Yet even though there are differences regarding the exact mechanisms behind α-syn aggregation, autophagy dysfunction seems to be involved in all three clinically distinct diseases, i.e., PD, DLB, and MSA.
2. Autophagic Dysregulation in Neuronal ASPs
PD is a common neurodegenerative disease, second after Alzheimer’s disease (AD) for incidence and affecting approximately 2–3% of the world’s population over the age of 65 [46,47]. PD symptoms include motor dysfunctions such as resting tremor, rigidity, and bradykinesia, as well as non-motor manifestations like cognitive impairment, mood, sleep disorders, and pain [48,49]. In particular, motor dysfunctions are the consequence of neuronal death in the substantia nigra (SN). Dopaminergic neuronal loss and accumulation of LB with fibrillar α-syn-aggregates represent the major well-established hallmarks of PD. Therapeutic approaches include treatments to alleviate symptoms or delay the worsening of the clinical phenotype, mainly based on long-term dopamine-based drugs like levodopa. However, to date, no treatment is reported to stop or reverse the progression of the disease [50].
DLB is mainly characterized by the presence of diffuse LBs in the SN, the cerebral cortex, and the subcortical nuclei, with a more marked cortex pathology compared with PD [51]. However, PD and DLB share many similarities in LBs’ occurrence and localization and can be distinguished mainly thanks to diagnostic criteria. In particular, clinical manifestations of DLB include cognitive deficits associated with a Parkinsonian phenotype, and fluctuations in attention and awareness, often accompanied by visual hallucinations [52,53]. The treatment of DLB combines the use of levodopa for Parkinsonism with cholinergic therapy to alleviate cognitive impairment [54], yet no treatments exist thus far that could halt the progression of DLB. As described in the introduction, autophagy is believed to be a major contributor regarding the clearance of aggregates of mutant proteins. However, the autophagic mechanisms involved in the pathogenesis and progression of DLB and PD remain ambiguous. To date, there is no study reporting that PD and DLB share the same pathogenic process; nonetheless, many common features have been observed in patients in the context of autophagy, as will be described below. While early reports investigated the involvement of the proteasome system in the degradation of α-syn aggregates [55], it is widely accepted nowadays that alterations in the autophagic pathways may be preferentially involved in neurodegenerative diseases, including PD [56].
2.1. Autophagy Involvement in Human Pathology of PD and DLB
The first indication that autophagy plays a role in neurodegeneration arose from several observations of accumulated autophagosomes in the brains of patients affected by diverse disorders, including PD, AD, and Huntington’s disease. Signs of autophagic degradation were detected in nigral neurons of PD patients; in particular, aggregated autophagosomes and aberrant lysosomes were found in post-mortem PD samples [56,57]. Furthermore, it was observed that, in LBs, α-syn colocalized with the autophagy marker LC3, and LC3-II levels were elevated in brain tissue from PD patients [58,59]. Along with the aforementioned markers, p62 also was detected in LBs from patients, further supporting the involvement of macroautophagy in the progression of PD [60]. Interestingly, in post-mortem brains from PD and DLB patients, it was observed that numerous upstream autophagosomal proteins, namely, ULK1, ULK2, Beclin-1, VPS34, and AMBRA1, are included in LBs and up-regulated in PD [61]. Another study with human brain samples showed that levels of the lysosomal type 5 P-type ATPase ATP13A2 were decreased in PD neurons, with ATP13A2 being sequestered in LBs [62]. ATP13A2 was reported to participate in transmembrane lysosomal transport and mutations affecting the ATP13A2 gene are linked to autosomal recessive familial PD [62,63]. In a recent study, the authors showed, using super-resolution microscopy, the existence of a crowded environment of organelles in PD patients’ brains, highlighting the presence of mitochondria, lysosomes, and autophagosomes [64].
Besides, in the context of CMA, samples from SN of PD patients displayed a selective loss of CMA markers, namely HSC70 protein and Lamp-2A [59,65]. Interestingly, one study showed that Lamp-2A levels are reduced concomitantly with the increase in α-syn levels at early stages of PD, suggesting that impairments in the CMA machinery could occur even before the large accumulation of α-syn takes place [65]. It has been shown that monomers and dimers of α-syn are mainly degraded by CMA compared with the oligomeric form [31,66]. Therefore, when the CMA machinery is compromised, monomers and dimers of α-syn tend to further aggregate, giving rise to even more oligomers. This hypothesis is consistent with several independent observations reporting that impaired CMA results in α-syn aggregation. This is true, for example, in the presence of mutations affecting proteins such as leucine-rich repeat kinase 2 (LRRK2), ubiquitin C-terminal hydrolase L1 (UCHL1), and DJ-1 (PARK7), all of which are responsible for the onset of familial forms of PD and can be degraded by CMA [59,66,67,68]. One study showed that PD brains with mutant LRRK2 protein create a self-perpetuating inhibitory effect on CMA [69]. Additionally, the involvement of microRNAs (miRNAs) in the regulation of CMA in PD has also been reported. The analysis of PD patients’ brains showed that six miRNAs predicted to regulate Lamp-2A and HSC70 were increased in the SN of patients, suggesting that the miRNA-induced dysregulation of the autophagic machinery plays an important role in PD pathogenesis and may represent a novel therapeutic target [70].
Furthermore, both macroautophagy and CMA rely on the presence and function of lysosomal enzymes, which have been reported to be impaired in post-mortem brains of PD patients. In particular, patients with mutations affecting the glucocerebrosidase gene (GBA) present a decrease in the activity of the lysosomal β-glucocerebrosidase (GCase) enzyme in several brain areas [71]. Moreover, Chu Y. and colleagues reported that PD nigral neurons exhibit lower levels of the lysosome associated membrane protein 1 (LAMP1), the lysosomal hydrolase Cathepsin D (CatD), and heat shock protein 73 (HSP73) in comparison with control brains [72]. Interestingly, immunoreactivity of GCase along with α-mannosidase and β-mannosidase, other lysosomal enzymes, was diminished in PD patients’ cerebrospinal fluid (CSF) [73,74]. A reduction in other autophagy markers, such as LC3B, Lamp-2A, and Beclin-1, was also observed in the CSF from subjects with PD [75]. Finally, peripheral blood mononuclear cells (PBMCs) isolated from PD patients can also be used to detect the presence of biomarkers, exhibiting diminished levels of HSC70 and Lamp-2A along with an up-regulation of LC3, ULK1, Beclin-1, and AMBRA1 [76,77,78,79]. Interestingly, a recent study investigated the methylation profile of ALP genes from the brains and appendices of PD patients, showing that abnormalities in these genes can also occur on an epigenetic level. In particular, hypermethylation of several genes, such as ULK1 and HSC70, was observed [80].
Post-mortem studies on DLB patients demonstrated that LC3 co-localizes with LBs in both the hippocampus and the temporal cortex [81,82]. Moreover, ultrastructural analyses showed the presence of abundant and abnormal autophagosomes [82]. In DLB brains, levels of mechanistic mTOR were increased and ATG7 levels were decreased. Interestingly, such alterations may be responsible for a defective initiation of the macroautophagy pathway, resulting in an impaired fusion of lysosomes and, consequently, accumulation of α-syn aggregates. Nonetheless, this might result in the formation of enlarged autophagic vacuole-like structures that have been previously observed by other groups as well (Table 1) [82].
Table 1.
Evidence of autophagy involvement found in post-mortem brain tissue of PD, DLB, and MSA patients.
| AutophagyMarkers | Function | PD | DLB | MSA |
|---|---|---|---|---|
| AMBRA1 | upstream regulator | increased immunoreactivity [61] | increased immunoreactivity [61] | increased protein levels and immunoreactivity [83] |
| ATG7 | upstream regulator | - | decreased protein levels and immunoreactivity [82] | - |
| ATP13A2 | lysosomal ATPase | decreased immunoreactivity [62] | - | - |
| Beclin-1 | autophagosome generation | increased protein levels [61] | partially increased protein levels [61] | increased immuno-reactivity [84] |
| Cathepsin D | lysosomal hydrolase | decreased immunoreactivity [72] | increased immunoreactivity [82] | - |
| HSC70 | chaperone involved in CMA | decreased protein levels [59] | - | increased immuno-reactivity [85] |
| Lamp-1 | lysosomal membrane glycoprotein | decreased immunoreactivity [72] | - | - |
| Lamp-2A | membrane receptor for CMA | decreased protein levels, immunoreactivity in few LBs [59,65] | - | - |
| LC3B | autophagosome generation | increased immunoreactivity [58], increased LC3B-II protein levels [59] | increased immunoreactivity [81,82] | increased immuno-reactivity [34,86] |
| mTOR | upstream regulator | - | increased protein levels and immunoreactivity [82] | - |
| p62 | upstream regulator | increased immunoreactivity [60] | - | increased immuno-reactivity [86] |
| ULK1/2 | upstream regulator | increased immunoreactivity [61] | increased immunoreactivity [61] | - |
| VPS34 | vesicle trafficking | increased immunoreactivity [61] | increased protein levels and immunoreactivity [61] | - |
The connection between PD and autophagy is further strengthened by several findings coming from in vivo and in vitro studies, which also help to shed light on the pathways involved in the onset and advancement of these disorders.
2.2. Analyses of Autophagy in PD Patient-Derived Induced Pluripotent Stem Cells
The use of induced pluripotent stem cells (iPSCs) enables the generation of human-specific models to study neuronal degeneration when PD-relevant genes are mutated [87,88,89,90,91,92]. The generation of midbrain dopaminergic neurons using iPSCs, an important step for studying neurodegenerative diseases, was accomplished following a protocol using bone morphogenetic protein (BMP)/SMAD inhibition and activation [93,94]. Notably, signs of autophagic impairment were observed in two independent studies in neuronal cells derived from iPSCs characterized by triplication of the SNCA locus [88,92]. Supplementary observations of a link between autophagy and PD come from studies involving the most frequent LRRK2 mutated variant, LRRK2-G2019S, which is subject to enhanced LRRK2 kinase activity. A novel study combined the use of iPS-derived neurons carrying the LRRK2-G2019S mutation and knock-in transgenic mice demonstrating that, in the presence of mutated LRRK2, the transport of autophagic vesicles along the axons is disrupted, mainly owing to hyper-phosphorylation of the Rab29 GTPase, an important regulator of trafficking [95].
2.3. Analyses of Autophagy in Mouse Models of PD and DLB
A mouse model carrying the α-syn A53T mutation has been described to show neuronal accumulation of α-syn and severe motor dysfunctions [96,97]. Interestingly, transgenic mice overexpressing mutant α-syn additionally exhibited an induced macroautophagic activity with elevated Beclin-1 and LC3-II levels [98]. Conditional knock-in mice expressing A53T in dopaminergic neurons only presented mutant α-syn localized on the mitochondrial membrane, thus leading to reduced activity of the mitochondrial complex I and raised levels of mitochondrial autophagy (mitophagy) [99]. In both a transgenic mouse model and a neuronal cell line, it was reported that co-overexpression of α-syn and Beclin-1 could ameliorate the phenotype and lead to a reduction in α-syn accumulation, suggesting that modulation of macroautophagy could partially compensate the CMA deregulation induced by α-syn [100].
In another study, the authors showed that mice lacking ATP13A2 develop motor symptoms and endolysosomal abnormalities, but no alterations in the accumulation and aggregation of α-syn are evident [101]. A few reports focused on the involvement of VPS35, a key player in vesicular trafficking, in the development of PD. Transgenic mice lacking VPS35 display α-syn accumulation and neuronal loss in the SN, occurring together with increased Lamp-2A degradation, reinforcing the suggestion that upstream autophagic proteins are also key regulators of α-syn aggregation and LB formation [102]. A LRRK2-G2019S knock-in mouse model and primary cells carrying this mutation displayed expanded lysosomes with decreased degradative activity and concomitant upregulation of ATP13A2 levels [103]. It was recently observed that the same mouse model is characterized by altered mitophagy, an impairment that is strongly correlated with familial PD in patients [104]. LRRK2 can also interact with p62, and it has been shown that p62 is essential for the turnover of LRRK2 mediated by autophagy [105].
A knockout model of DJ-1 in mice and knockdown in SH-SY5Y cells presented elevated α-syn aggregation and inhibition of the CMA machinery. This effect was mediated by DJ-1 selectively accelerating degradation of Lamp-2A in the lysosomes, hence destabilizing the autophagic pathway [67]. Furthermore, a recent study investigated the effect of overexpression of DJ-1 in a PD rotenone-induced mouse model. The authors showed that DJ-1 can act as a neuroprotective factor when its expression is increased in astrocytes, generating a reduction in the levels of α-syn and increased levels of Lamp-2A, thus suggesting a non-cell-autonomous function and potential therapeutic target for astrocytes in PD [106]. It has also been reported that microglial cells can act as a neuroprotector and mediate the degradation of α-syn through the so-called synucleinphagy, a selective autophagic mechanism. In particular, synucleinphagy requires an upregulation of p62 levels to function, and impairment of microglial autophagy in a mouse model overexpressing α-syn resulted in a greater accumulation of aggregates and increased death of dopaminergic neurons [107,108]. For more details on the interplay of neurons, microglia, and astrocytes in the context of PD, see the recent review of MacMahon Copas et al. [109].
It was recently reported that knock-in GbaL444P/WT transgenic mice also present, together with α-syn accumulation, reduced mitochondrial dynamics and impaired mitophagy [110]. Additionally, accumulation of α-syn was observed in a mouse model lacking another lysosomal enzyme, CatD. Remarkably, the same study reported that overexpression of CatD was able to rescue the phenotype by reducing α-syn aggregation in H4 neuroglioma cells, SH-SY5Y, and C. elegans [111].
Mutations in autophagy-related genes were also shown to play a role in α-syn accumulation in neuronal cells of various mouse models [112,113,114,115,116]. Transgenic Atg7 deficient mice showed autophagic impairment and progressive loss of neurons in the SN and striatum, along with increased α-syn and LRRK2 levels at the pre-synaptic compartment [114]. Moreover, knockout mice showed axonal dystrophy, highlighting a function of Atg7 in the trafficking of autophagic vesicles in the axons [112]. In the context of DLB, it has been proposed that a reduction in Atg7 and rise in mTOR levels could participate in the early phases of DLB; however, the detailed mechanisms remain to be elucidated [52].
2.4. Analyses of Autophagy in Different Cellular Models of PD and DLB
The first in vitro evidence of a connection between PD and autophagy came from a rat pheochromocytoma (PC12) cell line expressing either a mutant version of α-syn, A53T, or its wild type form. PC12 cells expressing mutant α-syn displayed an altered morphology and accumulation of lysosomal vacuolar structures, accompanied by observations of reduced lysosomal functions [117]. Other studies using the same cell line reported that wild type α-syn could be degraded through autophagy [28], especially through CMA, whereas the mutant form A53T failed to be degraded by CMA despite its high affinity for Lamp-2A. Thus, this resulted in an obstruction of the uptake and degradation of other substrates and triggered the activation of macroautophagy as a compensatory mechanism [118]. A similar phenotype was also observed using a human neuronal cell line [119]. Similarly to the mouse model described above, overexpressing Beclin-1 in PC12 cells carrying either the A53T or the A30P α-syn mutation resulted in a rescue of the phenotype and reduced α-syn accumulation [120]. In a recent study, midbrain dopaminergic neurons transfected with the A30P α-syn mutation exhibited a decreased autophagic activity shown by significantly elevated levels of p62 [30]. CMA can also be selectively blocked in the presence of α-syn post-translational modifications, as shown in the case of dopamine-modified α-syn in SH-SY5Y and mouse primary cells [121].
Yang Q. et al. investigated the role of myocyte enhancer factor 2D (MEF2D), a transcription factor associated with neuronal survival, in relation to autophagy and PD. It was observed that MEF2D can be degraded by CMA and, therefore, its levels were increased in a transgenic mouse model overexpressing α-syn, similar to samples derived from PD brains, thus indicating a direct connection between CMA regulation and neuronal survival [122]. As described before, altered levels of ATP13A2 were also reported in post-mortem PD samples, hence raising questions about the link between ATP13A2 and α-syn. In vitro knockdown of ATP13A2 in primary cortical neurons resulted in reduced lysosomal activity and consequent accumulation of α-syn, leading to elevated neuronal toxicity [123].
Along with LRRK2, DJ-1/PARK7 was also shown to be degraded by CMA in HEK293 cells and in a mouse dopaminergic progenitor SN4741 cell line [124]. Notably, studies reported a connection between DJ-1 and mitochondria. Knockdown of DJ-1 in M17 human dopaminergic neuroblastoma cells resulted in oxidative stress and compromised mitochondrial dynamics together with an increase in LC3-II levels, thus suggesting that DJ-1 might play a role in the modulation of autophagy in response to the presence of reactive oxygen species (ROS) [125].
As described before, GCase1 is a lysosomal enzyme acting downstream in the autophagic pathway. Nonetheless, it was observed that mutations in GBA1 in a neuroblastoma cell line are sufficient to induce an accumulation of α-syn and impaired lysosomal functions [126]. Analogous results were obtained in primary cells lentivirally transduced with a short hairpin RNA (shRNA) for knocking down GCase1 [127].
Taken together, in the past two decades, a multitude of studies are indicating that both macroautophagy and CMA participate in the onset and progression of PD and DLB. Impairment of the autophagic machinery was first observed in post-mortem samples from patients manifesting with an accumulation of vesicular structures and altered levels of various autophagy markers. The molecular and biological functions of such proteins have been extensively studied in vivo and in vitro. Remarkably, it seems that the accumulation of α-syn and the defective autophagic machinery are involved in a self-amplifying loop that drives the progression and worsening of the disease. In particular, accumulating evidence suggests that CMA has a higher impact on neuronal α-syn aggregation mechanisms compared with macroautophagy (Figure 2). However, many of the mechanisms regulating the interplay between autophagy-related proteins and PD-relevant genes remain to be elucidated. Therefore, autophagy not only represents an important aspect to study new pathogenic mechanisms of PD, but also offers the possibility of investigating autophagy modulation as a novel therapeutic target for neurodegenerative disorders.
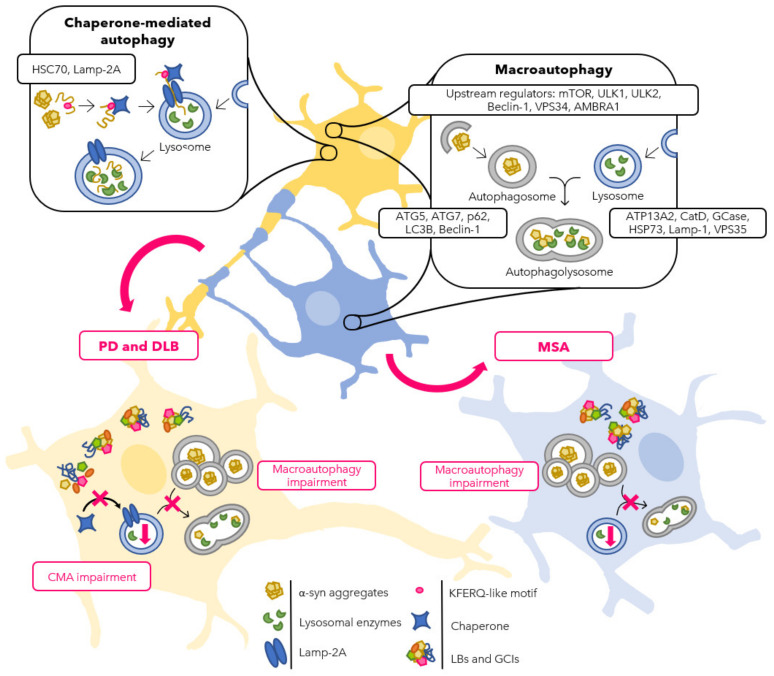
Figure 2.
Schematic representation of the autophagic flux in neurons (yellow) and oligodendrocytes (blue) in normal and PD, DLB, and MSA conditions. Physiologically, both CMA and macroautophagy pathways are involved in protein degradation in the cytoplasm of neurons, whereas macroautophagy seems to be the more effective mechanism in oligodendrocytes. It has been reported that, in the context of ASPs, an impairment of the autophagic machinery is involved. PD and DLB neurons (bottom left) present with LBs in the cytoplasm accumulating α-syn together with other proteins that can be degraded by neither macroautophagy nor CMA, such as LRRK2, DJ-1, and UCHL1. Moreover, other autophagy-related proteins, such as AMBRA1, p62, and LC3B, accumulate in LBs. Eventually, macroautophagy impairment results in an accumulation of autophagosomes and reduction in lysosomal activity. MSA oligodendrocytes (bottom right) show defects as well in the macroautophagic pathway with accumulation of GCIs containing α-syn, AMBRA1, p62, LC3B, and HSC70, together with an increased number of autophagosomes and diminished degradation in the lysosomes.
3. The Role of Autophagy in Oligodendroglial ASPs
MSA is an adult-onset, highly progressive, rare, but fatal neurodegenerative disease with unknown etiology. Clinically, MSA is defined by levodopa-unresponsive Parkinsonism, cerebellar ataxia, pyramidal signs, and autonomic failure in various combinations [128]. To date, no curative treatment for MSA is available; only symptomatic alleviation is possible regarding the current treatment options. Stopping the progression of MSA is still an elaborate task, as the mechanisms of disease progression are far from elucidated.
MSA is suggested to be a primary oligodendrogliopathy, as α-syn accumulates essentially in the cytoplasm of oligodendroglial cells, creating so-called GCIs. The oligodendroglial injury caused by these aggregates may be a leading cause of neurodegeneration, as the damaged oligodendroglial cells may no longer properly support neurons [129]. The development of α-syn-positive GCIs is far from elucidated so far; thus, the focus of research lies on the mechanisms behind it. Different hypotheses on the emergence of α-syn and these deposits in oligodendroglia exist. One possible explanation is the cumulative expression of α-syn in oligodendroglial cells and, later, the accumulation of the protein in the cytoplasm. However, the expression profile of SNCA in oligodendroglia is reported differently in the literature. On one hand, reports exist claiming that SNCA mRNA, and thus the protein, is present in oligodendroglia [130,131]. On the other hand, some studies could not observe oligodendroglial SNCA mRNA in MSA brains or only a transient expression of α-syn during oligodendroglial maturation [132,133]. The incorporation of extracellular neuronal-derived or recombinant α-syn by oligodendroglial cells has been reported several times in various in vitro and in vivo studies, also suggesting several mechanisms of uptake including endocytosis via clathrin or exosomes [131,134,135,136,137,138,139,140,141,142,143]. Prion-like transmission is another proposed mechanism that might lead to the accumulation of toxic α-syn in oligodendroglial cells, suggesting that α-syn behaves like a prion, thereby inducing MSA. However, in no study did significant amounts of α-syn-positive oligodendroglial inclusions occur; mostly, neuronal aggregates developed when adding α-syn from MSA brains to α-syn transgenic mice. Interestingly, prion-like behaviour of MSA brain homogenates could only be detected in mice having an A53T mutation, which is an α-syn mutation that occurs in PD patients and does not explain how GCIs and MSA develop driven by a prion-like manner [144,145,146]. Furthermore, α-syn seems to lack other different properties of a real prion protein, among others, the human transmission of α-syn, suggesting that MSA is not a real “prionopathy” [147,148].
3.1. Autophagy Involvement in Human Pathology of MSA
As discussed already in the introduction, in the cell, misfolded α-syn is degraded preferentially via the autophagy machinery [31,86,149]. If the macroautophagic machinery is not working appropriately, it can result in the aggregation of α-syn and, furthermore, in the formation of GCIs in oligodendroglial cells. In MSA brain tissue, numerous GCIs were found to be immunoreactive for LC3, a marker of macroautophagy. Thus, these GCIs were not positively stained for γ-aminobutyric-acid type A receptor-associated proteins (GABARAPs), suggesting a reduced maturation of autophagosomes [34]. In another study using MSA brain tissue, GCIs were stained positively for LC3, as well as for the ubiquitin-binding protein p62 and ubiquitin, confirming an upregulation of macroautophagy in oligodendroglial cells in MSA [149]. In a case report, p62-positive GCIs were present in MSA brain tissue of a patient with a prolonged clinical course [150]. Another case report claimed an increased amount of Beclin-1 corresponding to the area of oligodendroglial cell loss [84]. Numerous GCIs were positively stained for HSC70 in MSA compared with healthy brains in a different study by Kawamoto and colleagues [85]. The integration of autophagic proteins in GCIs indicates that the cell is starting an autophagy pathway and trying to clear the increased amount of α-syn, which is a cytoprotective mechanism [151]. Yet, the cell is not able to cope with the large amounts of toxic protein, leading to a reduction in autophagy proteins involved in downstream autophagic events, suggesting a defective macroautophagy maturation or suppressing CMA.
In a recent study, Miki Y. and colleagues proposed that upstream autophagy proteins also play a role in MSA pathogenesis. They could show that the autophagy/beclin1 regulator 1 (AMBRA1), an upstream protein of macroautophagy, is present in GCIs in brains of MSA patients, and that AMBRA1 silencing in neuronal cells leads to α-syn aggregate formation. It is suggested that AMBRA1 is connected to the recognition of abnormal and phosphorylated α-syn, hence activating autophagy to degrade these inclusions [83]. It is possible that the accumulation of AMBRA1 in GCIs might occur, trying to get rid of the large amount of α-syn by the cell, yet exceeding the cellular efficiency. Another mechanism involved in regulating macroautophagy has been proposed in another study, describing an increase in microRNA-101 (miR-101), among others, which targets different autophagy-related genes including RAB5A as well as MTOR, combined with a downregulation of the target gene RAB5A in the striatum of MSA patients [152]. Overexpression of miR-101 led to deficits in the autophagy machinery and, therefore, to accumulation of α-syn in oligodendroglial cells, substantiating the role for autophagy involvement in aggregation formation in MSA. The authors suggest that a dysregulation of miRNAs leads to a decreased expression of autophagy proteins, augmenting the accumulation of abnormal α-syn [152].
3.2. Analyses of Autophagy in Human-Derived Induced Pluripotent Stem Cells
The application of iPSCs was utilized to generate a human MSA iPSC model using human fibroblasts [132,153,154]. In the human MSA iPSC model, the authors could show a higher amount of the autophagic protein LC3-II in MSA patient-derived compared with control iPSC-derived neuronal cells. Furthermore, a decrease in the LC3-II flux was found in MSA-patient-derived iPSC, suggesting a disrupted autophagic flow, yet only in neuronal cells [154]. Even though these results were only shown in neuronal cells, this could be an indication for an overall disrupted autophagic machinery in MSA.
3.3. Analyses of Autophagy in Mouse Models of MSA
Using a transgenic MSA mouse model with overexpression of human α-syn under the oligodendroglial proteolipid protein promoter (PLP) [155], the levels of the macroautophagy protein LC3 were again increased when compared with wild type mice [156]. In another MSA mouse model, overexpressing α-syn under the myelin basic protein (MBP) promoter, an anti-miR-101 construct was delivered lentivirally into the striatum, enhancing macroautophagy, thereby decreasing the accumulation of α-syn in oligodendroglial cells [152]. The outcomes of these animal studies also suggest an involvement of macroautophagy in the aggregation mechanism of α-syn in oligodendroglial cells in MSA.
3.4. Analyses of Autophagy in Different Cellular Models of MSA
Different cell culture studies using oligodendroglial cells could also indicate an involvement of macroautophagy in aggregation formation of α-syn. Proteasomal inhibition in rat brain oligodendroglial cells led to the aggregation of α-syn and to the recruitment of LC3 and p62 to these inclusions, implying a close connection between the proteasomal system and the macroautophagy machinery [149]. In vitro induced mitochondrial impairment, oxidative stress, or macroautophagy inhibition caused the aggregation of exogenously added α-syn in rat brain oligodendroglial cells and/or in an oligodendroglial cell line [139]. In a recent study, it was found that α-syn preformed fibrils can facilitate the increase of endogenous α-syn in oligodendroglial precursor cells (OPCs) owing to a deficient autophagy machinery. The macroautophagy deficiency was confirmed by showing an increase in p62 and the LC3-II/LC3-I ratio in these OPCs [131]. Yet, in another study, the pharmacological inhibition of macroautophagy, as well as the knockdown of LC3 in an oligodendroglial cell line, only had limited effects on the accumulation formation of α-syn [136]. However, this outcome might correlate with the limited incubation time of oligodendroglial cells to extracellular α-syn species, because, in a different study, the uptake of α-syn from the extracellular space was suggested to be time-dependent [142]. These data suggest that, possibly, different forms of stress, including proteasomal, mitochondrial, oxidative, and autophagic stress, might be interconnected and must occur to create the full-blown pathology in MSA.
Summarizing, the afore-mentioned studies strengthen the assumption that an involvement of the autophagic pathway takes place in the initiation and progression of α-syn inclusion formation and, therefore, in disease progression in MSA. Although robust data on CMA regarding the recognition and degradation of α-syn in neuronal cells exist [29,118,121], only one human post-mortem study was published on an involvement of CMA in oligodendroglial inclusion formation in MSA so far, showing GCIs positively immunostained for HSC70 [85]. The available results introduce the speculation that HSC70 tries to bind and escort α-syn to the lysosome in oligodendroglial cells in MSA; however, it is not able to do so, thereby blocking CMA in its early stages, thus macroautophagy might be the more active degradation pathway in oligodendroglia (Figure 2). Yet, further studies will be needed, especially on CMA, to deepen and substantiate the current knowledge on the role of autophagy in GCI formation in oligodendroglial cells in MSA.
4. Concluding Remarks
To date, the involvement of autophagy in neurodegenerative diseases is well-acknowledged. As the pathological hallmark of most neurodegenerative diseases is the accumulation of different proteins in neuronal or glial cells, an impairment in the degradation process of these proteins is highly likely. Especially, in ASPs, the aggregation of α-syn and other proteins leads to the pathological hallmarks, which are LBs in PD and DLB and GCIs in MSA, initiating and/or progressing disease advancement. Macroautophagy and CMA have been shown to be major mechanisms involved in the attempt to reduce misfolded and aggregated α-syn in neuronal cells. In PD and DLB, evidence on the disturbances of macroautophagy and CMA was found in multiple studies. In MSA, a proven involvement of CMA in the inclusion formation of α-syn is still missing, yet different studies suggest a contribution of macroautophagy in disease initiation and progression. However, it is possible that, in oligodendroglial cells, CMA is repressed early when accumulated α-syn needs to be degraded. One possible mechanism could be that CMA is dependent on the form of the aggregated α-syn. Various toxic oligomeric α-syn variants were detected in PD brains [157,158] and different α-syn strains were described to result in different ASPs [159]. It could be that these different variants might also induce different pathways of degradation in PD, DLB and MSA, as well as in neurons and oligodendroglial cells. However, more studies will be needed to elucidate an overall picture on the role of macroautophagy and CMA in ASPs. If defined autophagic mechanisms or dysfunctions can be described, these studies will provide ideal candidates for drug development and, furthermore, might be involved in halting the progression of these neurodegenerative diseases.
Author Contributions
Conceptualization, L.F.; data curation, L.F. and E.G.; writing—original draft preparation, E.G. and L.F.; writing—review and editing, F.E., J.H., L.F. and E.G.; visualization, L.F., E.G. and F.E.; supervision, L.F., F.E. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Austrian Science Fund (FWF: I 4791-B and SFB F7810) and the German Research Foundation (DFG; ED 79/4-1) to F.E. Open Access Funding by the Austrian Science Fund (FWF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kupsky W.J., Grimes M.M., Sweeting J., Bertsch R., Cote L.J. Parkinson’s Disease and Megacolon. Neurology. 1987;37:1253. doi: 10.1212/WNL.37.7.1253. [DOI] [PubMed] [Google Scholar]
- 2.Wakabayashi K., Takahashi H., Takeda S., Ohama E., Ikuta F. Parkinson’s Disease: The Presence of Lewy Bodies in Auerbach’s and Meissner’s Plexuses. Acta Neuropathol. 1988;76:217–221. doi: 10.1007/BF00687767. [DOI] [PubMed] [Google Scholar]
- 3.Qualman S.J., Haupt H.M., Yang P., Hamilton S.R. Esophageal Lewy Bodies Associated with Ganglion Cell Loss in Achalasia: Similarity to Parkinson’s Disease. Gastroenterology. 1984;87:848–856. doi: 10.1016/0016-5085(84)90079-9. [DOI] [PubMed] [Google Scholar]
- 4.Spillantini M.G., Goedert M. The α-Synucleinopathies: Parkinson’s Disease, Dementia with Lewy Bodies, and Multiple System Atrophy. Ann. N. Y. Acad. Sci. 2000;920:16–27. doi: 10.1111/j.1749-6632.2000.tb06900.x. [DOI] [PubMed] [Google Scholar]
- 5.Baba M., Nakajo S., Tu P.H., Tomita T., Nakaya K., Lee V.M., Trojanowski J.Q., Iwatsubo T. Aggregation of Alpha-Synuclein in Lewy Bodies of Sporadic Parkinson’s Disease and Dementia with Lewy Bodies. Am. J. Pathol. 1998;152:879. [PMC free article] [PubMed] [Google Scholar]
- 6.Spillantini M.G., Schmidt M.L., Lee V.M.Y., Trojanowski J.Q., Jakes R., Goedert M. α-Synuclein in Lewy Bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 7.Papp M.I., Kahn J.E., Lantos P.L. Glial Cytoplasmic Inclusions in the CNS of Patients with Multiple System Atrophy (Striatonigral Degeneration, Olivopontocerebellar Atrophy and Shy-Drager Syndrome) J. Neurol. Sci. 1989;94:79–100. doi: 10.1016/0022-510X(89)90219-0. [DOI] [PubMed] [Google Scholar]
- 8.Trojanowski J.Q., Revesz T. Proposed Neuropathological Criteria for the Post Mortem Diagnosis of Multiple System Atrophy. Neuropathol. Appl. Neurobiol. 2007;33:615–620. doi: 10.1111/j.1365-2990.2007.00907.x. [DOI] [PubMed] [Google Scholar]
- 9.Dehay B., Martinez-Vicente M., Caldwell G.A., Caldwell K.A., Yue Z., Cookson M.R., Klein C., Vila M., Bezard E. Lysosomal Impairment in Parkinson’s Disease. Mov. Disord. 2013;28:725. doi: 10.1002/mds.25462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Manzoni C., Lewis P.A. Dysfunction of the Autophagy/Lysosomal Degradation Pathway Is a Shared Feature of the Genetic Synucleinopathies. FASEB J. 2013;27:3424–3429. doi: 10.1096/fj.12-223842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xilouri M., Brekk O.R., Stefanis L. Autophagy and Alpha-Synuclein: Relevance to Parkinson’s Disease and Related Synucleopathies. Mov. Disord. 2016;31:178–192. doi: 10.1002/mds.26477. [DOI] [PubMed] [Google Scholar]
- 12.Brück D., Wenning G.K., Stefanova N., Fellner L. Glia and Alpha-Synuclein in Neurodegeneration: A Complex Interaction. Neurobiol. Dis. 2016;85:262–274. doi: 10.1016/j.nbd.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fellner L., Irschick R., Schanda K., Reindl M., Klimaschewski L., Poewe W., Wenning G.K., Stefanova N. Toll-like Receptor 4 Is Required for α-Synuclein Dependent Activation of Microglia and Astroglia. Glia. 2013;61:349–360. doi: 10.1002/glia.22437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Halliday G.M., Stevens C.H. Glia: Initiators and Progressors of Pathology in Parkinson’s Disease. Mov. Disord. 2011;26:6–17. doi: 10.1002/mds.23455. [DOI] [PubMed] [Google Scholar]
- 15.Norris E.H., Giasson B.I., Lee V.M.Y. α-Synuclein: Normal Function and Role in Neurodegenerative Diseases. Curr. Top. Dev. Biol. 2004;60:17–54. doi: 10.1016/S0070-2153(04)60002-0. [DOI] [PubMed] [Google Scholar]
- 16.Iwai A., Masliah E., Yoshimoto M., Ge N., Flanagan L., Rohan de Silva H.A., Kittel A., Saitoh T. The Precursor Protein of Non-Aβ Component of Alzheimer’s Disease Amyloid Is a Presynaptic Protein of the Central Nervous System. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-X. [DOI] [PubMed] [Google Scholar]
- 17.Cheng F., Vivacqua G., Yu S. The Role of Alpha-Synuclein in Neurotransmission and Synaptic Plasticity. J. Chem. Neuroanat. 2011;42:242–248. doi: 10.1016/j.jchemneu.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W.H., Castillo P.E., Shinsky N., Garcia Verdugo J.M., Armanini M., Ryan A., et al. Mice Lacking α-Synuclein Display Functional Deficits in the Nigrostriatal Dopamine System. Neuron. 2000;25:239–252. doi: 10.1016/S0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 19.Hartman V.N., Miller M.A., Clayton D.F., Liu W.C., Kroodsma D.E., Brenowitz E.A. Testosterone Regulates α-Synuclein MRNA in the Avian Song System. Neuroreport. 2001;12:943–946. doi: 10.1097/00001756-200104170-00016. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen J.L., Dawson T.M., Dickson D.W., Petrucelli L. Caught in the Act: α-Synuclein Is the Culprit in Parkinson’s Disease. Neuron. 2003;40:453–456. doi: 10.1016/S0896-6273(03)00684-6. [DOI] [PubMed] [Google Scholar]
- 21.Dev K.K., Hofele K., Barbieri S., Buchman V.L., Van Der Putten H. Part II: α-Synuclein and Its Molecular Pathophysiological Role in Neurodegenerative Disease. Neuropharmacology. 2003;45:14–44. doi: 10.1016/S0028-3908(03)00140-0. [DOI] [PubMed] [Google Scholar]
- 22.Trexler A.J., Rhoades E. Single Molecule Characterization of α-Synuclein in Aggregation-Prone States. Biophys. J. 2010;99:3048. doi: 10.1016/j.bpj.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giasson B.I., Murray I.V.J., Trojanowski J.Q., Lee V.M.Y. A Hydrophobic Stretch of 12 Amino Acid Residues in the Middle of α-Synuclein Is Essential for Filament Assembly. J. Biol. Chem. 2001;276:2380–2386. doi: 10.1074/jbc.M008919200. [DOI] [PubMed] [Google Scholar]
- 24.Doherty C.P.A., Ulamec S.M., Maya-Martinez R., Good S.C., Makepeace J., Khan G.N., van Oosten-Hawle P., Radford S.E., Brockwell D.J. A Short Motif in the N-Terminal Region of α-Synuclein Is Critical for Both Aggregation and Function. Nat. Struct. Mol. Biol. 2020;27:249–259. doi: 10.1038/s41594-020-0384-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehay B., Bourdenx M., Gorry P., Przedborski S., Vila M., Hunot S., Singleton A., Olanow C.W., Merchant K.M., Bezard E., et al. Targeting α-Synuclein for Treatment of Parkinson’s Disease: Mechanistic and Therapeutic Considerations. Lancet Neurol. 2015;14:855–866. doi: 10.1016/S1474-4422(15)00006-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bieschke J., Russ J., Friedrich R.P., Ehrnhoefer D.E., Wobst H., Neugebauer K., Wanker E.E. EGCG Remodels Mature α-Synuclein and Amyloid-β Fibrils and Reduces Cellular Toxicity. Proc. Natl. Acad. Sci. USA. 2010;107:7710–7715. doi: 10.1073/pnas.0910723107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Auluck P.K., Chan H.Y.E., Trojanowski J.Q., Lee V.M.Y., Bonini N.M. Chaperone Suppression of α-Synuclein Toxicity in a Drosophila Model for Parkinson’s Disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 28.Webb J.L., Ravikumar B., Atkins J., Skepper J.N., Rubinsztein D.C. α-Synuclein Is Degraded by Both Autophagy and the Proteasome. J. Biol. Chem. 2003;278:25009–25013. doi: 10.1074/jbc.M300227200. [DOI] [PubMed] [Google Scholar]
- 29.Vogiatzi T., Xilouri M., Vekrellis K., Stefanis L. Wild Type α-Synuclein Is Degraded by Chaperone-Mediated Autophagy and Macroautophagy in Neuronal Cells. J. Biol. Chem. 2008;283:23542–23556. doi: 10.1074/jbc.M801992200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei Z., Cao G., Wei G. A30P Mutant α-Synuclein Impairs Autophagic Flux by Inactivating JNK Signaling to Enhance ZKSCAN3 Activity in Midbrain Dopaminergic Neurons. Cell Death Dis. 2019;10:133. doi: 10.1038/s41419-019-1364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Vicente M., Vila M. Alpha-Synuclein and Protein Degradation Pathways in Parkinson’s Disease: A Pathological Feed-Back Loop. Exp. Neurol. 2013;247:308–313. doi: 10.1016/j.expneurol.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Tanji K., Mori F., Kakita A., Takahashi H., Wakabayashi K. Alteration of Autophagosomal Proteins (LC3, GABARAP and GATE-16) in Lewy Body Disease. Neurobiol. Dis. 2011;43:690–697. doi: 10.1016/j.nbd.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 33.Chang D., Nalls M.A., Hallgrímsdóttir I.B., Hunkapiller J., van der Brug M., Cai F., Kerchner G.A., Ayalon G., Bingol B., Sheng M., et al. A Meta-Analysis of Genome-Wide Association Studies Identifies 17 New Parkinson’s Disease Risk Loci. Nat. Genet. 2017;49:1511–1516. doi: 10.1038/ng.3955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanji K., Odagiri S., Maruyama A., Mori F., Kakita A., Takahashi H., Wakabayashi K. Alteration of Autophagosomal Proteins in the Brain of Multiple System Atrophy. Neurobiol. Dis. 2013;49:190–198. doi: 10.1016/j.nbd.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Chiba Y., Takei S., Kawamura N., Kawaguchi Y., Sasaki K., Hasegawa-Ishii S., Furukawa A., Hosokawa M., Shimada A. Immunohistochemical Localization of Aggresomal Proteins in Glial Cytoplasmic Inclusions in Multiple System Atrophy. Neuropathol. Appl. Neurobiol. 2012;38:559–571. doi: 10.1111/j.1365-2990.2011.01229.x. [DOI] [PubMed] [Google Scholar]
- 36.Hou X., Watzlawik J.O., Fiesel F.C., Springer W. Autophagy in Parkinson’s Disease. J. Mol. Biol. 2020;432:2651–2672. doi: 10.1016/j.jmb.2020.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsukada M., Ohsumi Y. Isolation and Characterization of Autophagy-Defective Mutants of Saccharomyces Cerevisiae. FEBS Lett. 1993;333:169–174. doi: 10.1016/0014-5793(93)80398-E. [DOI] [PubMed] [Google Scholar]
- 38.Kabeya Y., Mizushima N., Ueno T., Yamamoto A., Kirisako T., Noda T., Kominami E., Ohsumi Y., Yoshimori T. LC3, a Mammalian Homologue of Yeast Apg8p, Is Localized in Autophagosome Membranes after Processing. EMBO J. 2000;19:5720–5728. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pankiv S., Clausen T.H., Lamark T., Brech A., Bruun J.A., Outzen H., Øvervatn A., Bjørkøy G., Johansen T. P62/SQSTM1 Binds Directly to Atg8/LC3 to Facilitate Degradation of Ubiquitinated Protein Aggregates by Autophagy*. J. Biol. Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- 40.Bjørkøy G., Lamark T., Brech A., Outzen H., Perander M., Øvervatn A., Stenmark H., Johansen T. P62/SQSTM1 Forms Protein Aggregates Degraded by Autophagy and Has a Protective Effect on Huntingtin-Induced Cell Death. J. Cell Biol. 2005;171:603–614. doi: 10.1083/jcb.200507002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Damme M., Suntio T., Saftig P., Eskelinen E.L. Autophagy in Neuronal Cells: General Principles and Physiological and Pathological Functions. Acta Neuropathol. 2015;129:337–362. doi: 10.1007/s00401-014-1361-4. [DOI] [PubMed] [Google Scholar]
- 42.Dikic I., Elazar Z. Mechanism and Medical Implications of Mammalian Autophagy. Nat. Rev. Mol. Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- 43.Yang Z., Klionsky D.J. Mammalian Autophagy: Core Molecular Machinery and Signaling Regulation. Curr. Opin. Cell Biol. 2010;22:124–131. doi: 10.1016/j.ceb.2009.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dice J.F. Peptide Sequences That Target Cytosolic Proteins for Lysosomal Proteolysis. Trends Biochem. Sci. 1990;15:305–309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 45.Cuervo A.M., Dice J.F. A Receptor for the Selective Uptake and Degradation of Proteins by Lysosomes. Science. 1996;273:501–503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 46.Obeso J.A., Stamelou M., Goetz C.G., Poewe W., Lang A.E., Weintraub D., Burn D., Halliday G.M., Bezard E., Przedborski S., et al. Past, Present, and Future of Parkinson’s Disease: A Special Essay on the 200th Anniversary of the Shaking Palsy. Mov. Disord. 2017;32:1264–1310. doi: 10.1002/mds.27115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Poewe W., Seppi K., Tanner C.M., Halliday G.M., Brundin P., Volkmann J., Schrag A.E., Lang A.E. Parkinson Disease. Nat. Rev. Dis. Prim. 2017;3:17013. doi: 10.1038/nrdp.2017.13. [DOI] [PubMed] [Google Scholar]
- 48.Poewe W. Non-Motor Symptoms in Parkinson’s Disease. Eur. J. Neurol. 2008;15((Suppl. 1)):S14–S20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 49.Schapira A.H.V., Chaudhuri K.R., Jenner P. Non-Motor Features of Parkinson Disease. Nat. Rev. Neurosci. 2017;18:435–450. doi: 10.1038/nrn.2017.62. [DOI] [PubMed] [Google Scholar]
- 50.Stoker T.B., Torsney K.M., Barker R.A. Emerging Treatment Approaches for Parkinson’s Disease. Front. Neurosci. 2018;12:693. doi: 10.3389/fnins.2018.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McCann H., Stevens C.H., Cartwright H., Halliday G.M. α-Synucleinopathy Phenotypes. Parkinsonism Relat. Disord. 2014;20((Suppl. 1)):S62–S67. doi: 10.1016/S1353-8020(13)70017-8. [DOI] [PubMed] [Google Scholar]
- 52.Kragh C.L., Ubhi K., Wyss-Corey T., Masliah E. Autophagy in Dementias. Brain Pathol. 2012;22:99–109. doi: 10.1111/j.1750-3639.2011.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McKeith I.G., Boeve B.F., Dickson D.W., Halliday G., Taylor J.P., Weintraub D., Aarsland D., Galvin J., Attems J., Ballard C.G., et al. Diagnosis and Management of Dementia with Lewy Bodies. Neurology. 2017;89:88–100. doi: 10.1212/WNL.0000000000004058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zesiewicz T.A., Baker M.J., Dunne P.B., Hauser R.A. Diffuse Lewy Body Disease. Curr. Treat. Options Neurol. 2001;3:507–518. doi: 10.1007/s11940-001-0013-x. [DOI] [PubMed] [Google Scholar]
- 55.Tofaris G.K., Layfield R., Spillantini M.G. α-Synuclein Metabolism and Aggregation Is Linked to Ubiquitin-Independent Degradation by the Proteasome. FEBS Lett. 2001;509:22–26. doi: 10.1016/S0014-5793(01)03115-5. [DOI] [PubMed] [Google Scholar]
- 56.Levine B., Kroemer G. Autophagy in the Pathogenesis of Disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anglade P., Vyas S., Javoy-Agid F., Herrero M.T., Michel P.P., Marquez J., Mouatt-Prigent A., Ruberg M., Hirsch E.C., Agid Y. Apoptosis and Autophagy in Nigral Neurons of Patients with Parkinson’s Disease. Histol. Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- 58.Dehay B., Bove J., Rodriguez-Muela N., Perier C., Recasens A., Boya P., Vila M. Pathogenic Lysosomal Depletion in Parkinson’s Disease. J. Neurosci. 2010;30:12535–12544. doi: 10.1523/JNEUROSCI.1920-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alvarez-Erviti L., Rodriguez-Oroz M.C., Cooper J.M., Caballero C., Ferrer I., Obeso J.A., Schapira A.H.V. Chaperone-Mediated Autophagy Markers in Parkinson Disease Brains. Arch. Neurol. 2010;67:1464–1472. doi: 10.1001/archneurol.2010.198. [DOI] [PubMed] [Google Scholar]
- 60.Zatloukal K., Stumptner C., Fuchsbichler A., Heid H., Schnoelzer M., Kenner L., Kleinert R., Prinz M., Aguzzi A., Denk H. P62 Is a Common Component of Cytoplasmic Inclusions in Protein Aggregation Diseases. Am. J. Pathol. 2002;160:255–263. doi: 10.1016/S0002-9440(10)64369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miki Y., Tanji K., Mori F., Utsumi J., Sasaki H., Kakita A., Takahashi H., Wakabayashi K. Alteration of Upstream Autophagy-Related Proteins (ULK1, ULK2, Beclin1, VPS34 and AMBRA1) in Lewy Body Disease. Brain Pathol. 2016;26:359–370. doi: 10.1111/bpa.12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dehay B., Ramirez A., Martinez-Vicente M., Perier C., Canron M.H., Doudnikoff E., Vital A., Vila M., Klein C., Bezard E. Loss of P-Type ATPase ATP13A2/PARK9 Function Induces General Lysosomal Deficiency and Leads to Parkinson Disease Neurodegeneration. Proc. Natl. Acad. Sci. USA. 2012;109:9611–9616. doi: 10.1073/pnas.1112368109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ramirez A., Heimbach A., Gründemann J., Stiller B., Hampshire D., Cid L.P., Goebel I., Mubaidin A.F., Wriekat A.L., Roeper J., et al. Hereditary Parkinsonism with Dementia Is Caused by Mutations in ATP13A2, Encoding a Lysosomal Type 5 P-Type ATPase. Nat. Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- 64.Shahmoradian S.H., Lewis A.J., Genoud C., Hench J., Moors T.E., Navarro P.P., Castaño-Díez D., Schweighauser G., Graff-Meyer A., Goldie K.N., et al. Lewy Pathology in Parkinson’s Disease Consists of Crowded Organelles and Lipid Membranes. Nat. Neurosci. 2019;22:1099–1109. doi: 10.1038/s41593-019-0423-2. [DOI] [PubMed] [Google Scholar]
- 65.Murphy K.E., Gysbers A.M., Abbott S.K., Spiro A.S., Furuta A., Cooper A., Garner B., Kabuta T., Halliday G.M. Lysosomal-Associated Membrane Protein 2 Isoforms Are Differentially Affected in Early Parkinson’s Disease. Mov. Disord. 2015;30:1639–1647. doi: 10.1002/mds.26141. [DOI] [PubMed] [Google Scholar]
- 66.Rana T., Behl T., Sehgal A., Mehta V., Singh S., Bhatia S., Al-Harrasi A., Bungau S. Exploring the Role of Autophagy Dysfunction in Neurodegenerative Disorders. Mol. Neurobiol. 2021;58:4886–4905. doi: 10.1007/s12035-021-02472-0. [DOI] [PubMed] [Google Scholar]
- 67.Xu C.Y., Kang W.Y., Chen Y.M., Jiang T.F., Zhang J., Zhang L.N., Ding J.Q., Liu J., Chen S.D. DJ-1 Inhibits α-Synuclein Aggregation by Regulating Chaperone-Mediated Autophagy. Front. Aging Neurosci. 2017;9:308. doi: 10.3389/fnagi.2017.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sala G., Marinig D., Arosio A., Ferrarese C. Role of Chaperone-Mediated Autophagy Dysfunctions in the Pathogenesis of Parkinson’s Disease. Front. Mol. Neurosci. 2016;9:157. doi: 10.3389/fnmol.2016.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Orenstein S.J., Kuo S.H., Tasset I., Arias E., Koga H., Fernandez-Carasa I., Cortes E., Honig L.S., Dauer W., Consiglio A., et al. Interplay of LRRK2 with Chaperone-Mediated Autophagy. Nat. Neurosci. 2013;16:394–406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alvarez-Erviti L., Seow Y., Schapira A.H.V., Rodriguez-Oroz M.C., Obeso J.A., Cooper J.M. Influence of MicroRNA Deregulation on Chaperone-Mediated Autophagy and α-Synuclein Pathology in Parkinson’s Disease. Cell Death Dis. 2013;4:e545. doi: 10.1038/cddis.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gegg M.E., Burke D., Heales S.J.R., Cooper J.M., Hardy J., Wood N.W., Schapira A.H.V. Glucocerebrosidase Deficiency in Substantia Nigra of Parkinson Disease Brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chu Y., Dodiya H., Aebischer P., Olanow C.W., Kordower J.H. Alterations in Lysosomal and Proteasomal Markers in Parkinson’s Disease: Relationship to Alpha-Synuclein Inclusions. Neurobiol. Dis. 2009;35:385–398. doi: 10.1016/j.nbd.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 73.Balducci C., Pierguidi L., Persichetti E., Parnetti L., Sbaragli M., Tassi C., Orlacchio A., Calabresi P., Beccari T., Rossi A. Lysosomal Hydrolases in Cerebrospinal Fluid from Subjects with Parkinson’s Disease. Mov. Disord. 2007;22:1481–1484. doi: 10.1002/mds.21399. [DOI] [PubMed] [Google Scholar]
- 74.Parnetti L., Chiasserini D., Persichetti E., Eusebi P., Varghese S., Qureshi M.M., Dardis A., Deganuto M., De Carlo C., Castrioto A., et al. Cerebrospinal Fluid Lysosomal Enzymes and Alpha-Synuclein in Parkinson’s Disease. Mov. Disord. 2014;29:1019–1027. doi: 10.1002/mds.25772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Youn J., Lee S.B., Lee H.S., Yang H.O., Park J., Kim J.S., Oh E., Park S., Jang W. Cerebrospinal Fluid Levels of Autophagy-Related Proteins Represent Potentially Novel Biomarkers of Early-Stage Parkinson’s Disease. Sci. Rep. 2018;8:16866. doi: 10.1038/s41598-018-35376-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu G., Wang X., Feng X., Zhang A., Li J., Gu K., Huang J., Pang S., Dong H., Gao H., et al. Altered Expression of Autophagic Genes in the Peripheral Leukocytes of Patients with Sporadic Parkinson’s Disease. Brain Res. 2011;1394:105–111. doi: 10.1016/j.brainres.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 77.Sala G., Stefanoni G., Arosio A., Riva C., Melchionda L., Saracchi E., Fermi S., Brighina L., Ferrarese C. Reduced Expression of the Chaperone-Mediated Autophagy Carrier Hsc70 Protein in Lymphomonocytes of Patients with Parkinson’s Disease. Brain Res. 2014;1546:46–52. doi: 10.1016/j.brainres.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 78.Papagiannakis N., Xilouri M., Koros C., Simitsi A.M., Stamelou M., Maniati M., Stefanis L. Autophagy Dysfunction in Peripheral Blood Mononuclear Cells of Parkinson’s Disease Patients. Neurosci. Lett. 2019;704:112–115. doi: 10.1016/j.neulet.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 79.Miki Y., Shimoyama S., Kon T., Ueno T., Hayakari R., Tanji K., Matsumiya T., Tsushima E., Mori F., Wakabayashi K., et al. Alteration of Autophagy-Related Proteins in Peripheral Blood Mononuclear Cells of Patients with Parkinson’s Disease. Neurobiol. Aging. 2018;63:33–43. doi: 10.1016/j.neurobiolaging.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 80.Gordevicius J., Li P., Marshall L.L., Killinger B.A., Lang S., Ensink E., Kuhn N.C., Cui W., Maroof N., Lauria R., et al. Epigenetic Inactivation of the Autophagy–Lysosomal System in Appendix in Parkinson’s Disease. Nat. Commun. 2021;12:5134. doi: 10.1038/s41467-021-25474-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Higashi S., Moore D.J., Minegishi M., Kasanuki K., Fujishiro H., Kabuta T., Togo T., Katsuse O., Uchikado H., Furukawa Y., et al. Localization of MAP1-LC3 in Vulnerable Neurons and Lewy Bodies in Brains of Patients with Dementia with Lewy Bodies. J. Neuropathol. Exp. Neurol. 2011;70:264–280. doi: 10.1097/NEN.0b013e318211c86a. [DOI] [PubMed] [Google Scholar]
- 82.Crews L., Spencer B., Desplats P., Patrick C., Paulino A., Rockenstein E., Hansen L., Adame A., Galasko D., Masliah E. Selective Molecular Alterations in the Autophagy Pathway in Patients with Lewy Body Disease and in Models of α-Synucleinopathy. PLoS ONE. 2010;5:e9313. doi: 10.1371/journal.pone.0009313. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 83.Miki Y., Tanji K., Mori F., Tatara Y., Utsumi J., Sasaki H., Kakita A., Takahashi H., Fimia G.M., Wakabayashi K. AMBRA1, a Novel α-Synuclein-Binding Protein, Is Implicated in the Pathogenesis of Multiple System Atrophy. Brain Pathol. 2018;28:28–42. doi: 10.1111/bpa.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Benarroch E.E., Schmeichel A.M., Parisi J.E., Low P.A. Putative Neuropathological Interactions in MSA: Focus in the Rostral Ventrolateral Medulla. Clin. Auton. Res. 2015;25:77–80. doi: 10.1007/s10286-015-0273-2. [DOI] [PubMed] [Google Scholar]
- 85.Kawamoto Y., Akiguchi I., Shirakashi Y., Honjo Y., Tomimoto H., Takahashi R., Budka H. Accumulation of Hsc70 and Hsp70 in Glial Cytoplasmic Inclusions in Patients with Multiple System Atrophy. Brain Res. 2007;1136:219–227. doi: 10.1016/j.brainres.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 86.Ebrahimi-Fakhari D., Cantuti-Castelvetri I., Fan Z., Rockenstein E., Masliah E., Hyman B.T., McLean P.J., Unni V.K. Distinct Roles In Vivo for the Ubiquitin–Proteasome System and the Autophagy–Lysosomal Pathway in the Degradation of α-Synuclein. J. Neurosci. 2011;31:14508. doi: 10.1523/JNEUROSCI.1560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung C.Y., Khurana V., Auluck P.K., Tardiff D.F., Mazzulli J.R., Soldner F., Baru V., Lou Y., Freyzon Y., Cho S., et al. Identification and Rescue of α-Synuclein Toxicity in Parkinson Patient–Derived Neurons. Science. 2013;342:983–987. doi: 10.1126/science.1245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Oliveira L.M.A., Falomir-Lockhart L.J., Botelho M.G., Lin K.H., Wales P., Koch J.C., Gerhardt E., Taschenberger H., Outeiro T.F., Lingor P., et al. Elevated α-Synuclein Caused by SNCA Gene Triplication Impairs Neuronal Differentiation and Maturation in Parkinson’s Patient-Derived Induced Pluripotent Stem Cells. Cell Death Dis. 2015;6:e1994. doi: 10.1038/cddis.2015.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Barbuti P., Antony P., Santos B., Massart F., Cruciani G., Dording C., Arias J., Schwamborn J., Krüger R. Using High-Content Screening to Generate Single-Cell Gene-Corrected Patient-Derived IPS Clones Reveals Excess Alpha-Synuclein with Familial Parkinson’s Disease Point Mutation A30P. Cells. 2020;9:2065. doi: 10.3390/cells9092065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Suzuki H., Egawa N., Kondo T., Imamura K., Enami T., Tsukita K., Suga M., Shibukawa R., Okanishi Y., Uchiyama T., et al. Generation of a Human Induced Pluripotent Stem Cell Line Derived from a Parkinson’s Disease Patient Carrying SNCA Duplication. Stem Cell Res. 2020;45:101828. doi: 10.1016/j.scr.2020.101828. [DOI] [PubMed] [Google Scholar]
- 91.Devine M.J., Ryten M., Vodicka P., Thomson A.J., Burdon T., Houlden H., Cavaleri F., Nagano M., Drummond N.J., Taanman J.W., et al. Parkinson’s Disease Induced Pluripotent Stem Cells with Triplication of the α-Synuclein Locus. Nat. Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heman-Ackah S.M., Manzano R., Hoozemans J.J.M., Scheper W., Flynn R., Haerty W., Cowley S.A., Bassett A.R., Wood M.J.A. Alpha-Synuclein Induces the Unfolded Protein Response in Parkinson’s Disease SNCA Triplication IPSC-Derived Neurons. Hum. Mol. Genet. 2017;26:4441–4450. doi: 10.1093/hmg/ddx331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Jovanovic V.M., Salti A., Tilleman H., Zega K., Jukic M.M., Zou H., Friedel R.H., Prakash N., Blaess S., Edenhofer F., et al. BMP/SMAD Pathway Promotes Neurogenesis of Midbrain Dopaminergic Neurons In Vivo and in Human Induced Pluripotent and Neural Stem Cells. J. Neurosci. 2018;38:1662. doi: 10.1523/JNEUROSCI.1540-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Worsdorfer P., Thier M., Kadari A., Edenhofer F. Roadmap to Cellular Reprogramming—Manipulating Transcriptional Networks with DNA, RNA, Proteins and Small Molecules. Curr. Mol. Med. 2013;13:868–878. doi: 10.2174/1566524011313050017. [DOI] [PubMed] [Google Scholar]
- 95.Boecker C.A., Goldsmith J., Dou D., Cajka G.G., Holzbaur E.L.F. Increased LRRK2 Kinase Activity Alters Neuronal Autophagy by Disrupting the Axonal Transport of Autophagosomes. Curr. Biol. 2021;31:2140–2154. doi: 10.1016/j.cub.2021.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Giasson B.I., Duda J.E., Quinn S.M., Zhang B., Trojanowski J.Q., Lee V.M.Y. Neuronal Alpha-Synucleinopathy with Severe Movement Disorder in Mice Expressing A53T Human Alpha-Synuclein. Neuron. 2002;34:521–533. doi: 10.1016/S0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 97.Lee M.K., Stirling W., Xu Y., Xu X., Qui D., Mandir A.S., Dawson T.M., Copeland N.G., Jenkins N.A., Price D.L. Human α-Synuclein-Harboring Familial Parkinson’s Disease-Linked Ala-53 → Thr Mutation Causes Neurodegenerative Disease with α-Synuclein Aggregation in Transgenic Mice. Proc. Natl. Acad. Sci. USA. 2002;99:8968–8973. doi: 10.1073/pnas.132197599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yu W.H., Dorado B., Figueroa H.Y., Wang L., Planel E., Cookson M.R., Clark L.N., Duff K.E. Metabolic Activity Determines Efficacy of Macroautophagic Clearance of Pathological Oligomeric Alpha-Synuclein. Am. J. Pathol. 2009;175:736–747. doi: 10.2353/ajpath.2009.080928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chinta S.J., Mallajosyula J.K., Rane A., Andersen J.K. Mitochondrial α-Synuclein Accumulation Impairs Complex I Function in Dopaminergic Neurons and Results in Increased Mitophagy In Vivo. Neurosci. Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Spencer B., Potkar R., Trejo M., Rockenstein E., Patrick C., Gindi R., Adame A., Wyss-Coray T., Masliah E. Beclin 1 Gene Transfer Activates Autophagy and Ameliorates the Neurodegenerative Pathology in Alpha-Synuclein Models of Parkinson’s and Lewy Body Diseases. J. Neurosci. 2009;29:13578–13588. doi: 10.1523/JNEUROSCI.4390-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kett L.R., Stiller B., Bernath M.M., Tasset I., Blesa J., Jackson-Lewis V., Chan R.B., Zhou B., Di Paolo G., Przedborski S., et al. α-Synuclein-Independent Histopathological and Motor Deficits in Mice Lacking the Endolysosomal Parkinsonism Protein Atp13a2. J. Neurosci. 2015;35:5724–5742. doi: 10.1523/JNEUROSCI.0632-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tang F.L., Erion J.R., Tian Y., Liu W., Yin D.M., Ye J., Tang B., Mei L., Xiong W.C. VPS35 in Dopamine Neurons Is Required for Endosome-to-Golgi Retrieval of Lamp2a, a Receptor of Chaperone-Mediated Autophagy That Is Critical for α-Synuclein Degradation and Prevention of Pathogenesis of Parkinson’s Disease. J. Neurosci. 2015;35:10613–10628. doi: 10.1523/JNEUROSCI.0042-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Henry A.G., Aghamohammadzadeh S., Samaroo H., Chen Y., Mou K., Needle E., Hirst W.D. Pathogenic LRRK2 Mutations, through Increased Kinase Activity, Produce Enlarged Lysosomes with Reduced Degradative Capacity and Increase ATP13A2 Expression. Hum. Mol. Genet. 2015;24:6013–6028. doi: 10.1093/hmg/ddv314. [DOI] [PubMed] [Google Scholar]
- 104.Singh F., Prescott A.R., Rosewell P., Ball G., Reith A.D., Ganley I.G. Pharmacological Rescue of Impaired Mitophagy in Parkinson’s Disease-Related LRRK2 G2019S Knock-in Mice. Elife. 2021;10:e67604. doi: 10.7554/eLife.67604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Park S., Han S., Choi I., Kim B., Park S.P., Joe E.H., Suh Y.H. Interplay between Leucine-Rich Repeat Kinase 2 (LRRK2) and P62/SQSTM-1 in Selective Autophagy. PLoS ONE. 2016;11:e0163029. doi: 10.1371/journal.pone.0163029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.De Miranda B.R., Rocha E.M., Bai Q., El Ayadi A., Hinkle D., Burton E.A., Greenamyre J.T. Astrocyte-Specific DJ-1 Overexpression Protects against Rotenone-Induced Neurotoxicity in a Rat Model of Parkinson’s Disease. Neurobiol. Dis. 2018;115:101–114. doi: 10.1016/j.nbd.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi I., Seegobin S.P., Liang D., Yue Z. Synucleinphagy: A Microglial “Community Cleanup Program” for Neuroprotection. Autophagy. 2020;16:1718–1720. doi: 10.1080/15548627.2020.1774149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Choi I., Zhang Y., Seegobin S.P., Pruvost M., Wang Q., Purtell K., Zhang B., Yue Z. Microglia Clear Neuron-Released α-Synuclein via Selective Autophagy and Prevent Neurodegeneration. Nat. Commun. 2020;11:1386. doi: 10.1038/s41467-020-15119-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.MacMahon Copas A.N., McComish S.F., Fletcher J.M., Caldwell M.A. The Pathogenesis of Parkinson’s Disease: A Complex Interplay between Astrocytes, Microglia, and T Lymphocytes? Front. Neurol. 2021;12:771. doi: 10.3389/fneur.2021.666737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li H., Ham A., Ma T.C., Kuo S.H., Kanter E., Kim D., Ko H.S., Quan Y., Sardi S.P., Li A., et al. Mitochondrial Dysfunction and Mitophagy Defect Triggered by Heterozygous GBA Mutations. Autophagy. 2019;15:113–130. doi: 10.1080/15548627.2018.1509818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Qiao L., Hamamichi S., Caldwell K.A., Caldwell G.A., Yacoubian T.A., Wilson S., Xie Z.L., Speake L.D., Parks R., Crabtree D., et al. Lysosomal Enzyme Cathepsin D Protects against Alpha-Synuclein Aggregation and Toxicity. Mol. Brain. 2008;1:17. doi: 10.1186/1756-6606-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Komatsu M., Wang Q.J., Holstein G.R., Friedrich V.L., Iwata J., Kominami E., Chait B.T., Tanaka K., Yue Z. Essential Role for Autophagy Protein Atg7 in the Maintenance of Axonal Homeostasis and the Prevention of Axonal Degeneration. Proc. Natl. Acad. Sci. USA. 2007;104:14489–14494. doi: 10.1073/pnas.0701311104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Komatsu M., Waguri S., Chiba T., Murata S., Iwata J., Tanida I., Ueno T., Koike M., Uchiyama Y., Kominami E., et al. Loss of Autophagy in the Central Nervous System Causes Neurodegeneration in Mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 114.Friedman L.G., Lachenmayer M.L., Wang J., He L., Poulose S.M., Komatsu M., Holstein G.R., Yue Z. Disrupted Autophagy Leads to Dopaminergic Axon and Dendrite Degeneration and Promotes Presynaptic Accumulation of α-Synuclein and LRRK2 in the Brain. J. Neurosci. 2012;32:7585–7593. doi: 10.1523/JNEUROSCI.5809-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Nishiyama J., Miura E., Mizushima N., Watanabe M., Yuzaki M. Aberrant Membranes and Double-Membrane Structures Accumulate in the Axons of Atg5-Null Purkinje Cells before Neuronal Death. Autophagy. 2007;3:591–596. doi: 10.4161/auto.4964. [DOI] [PubMed] [Google Scholar]
- 116.Sheehan P., Yue Z. Deregulation of Autophagy and Vesicle Trafficking in Parkinson’s Disease. Neurosci. Lett. 2019;697:59–65. doi: 10.1016/j.neulet.2018.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Stefanis L., Larsen K.E., Rideout H.J., Sulzer D., Greene L.A. Expression of A53T Mutant but Not Wild-Type Alpha-Synuclein in PC12 Cells Induces Alterations of the Ubiquitin-Dependent Degradation System, Loss of Dopamine Release, and Autophagic Cell Death. J. Neurosci. 2001;21:9549–9560. doi: 10.1523/JNEUROSCI.21-24-09549.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cuervo A.M., Stafanis L., Fredenburg R., Lansbury P.T., Sulzer D. Impaired Degradation of Mutant α-Synuclein by Chaperone-Mediated Autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 119.Xilouri M., Vogiatzi T., Vekrellis K., Park D., Stefanis L. Abberant Alpha-Synuclein Confers Toxicity to Neurons in Part through Inhibition of Chaperone-Mediated Autophagy. PLoS ONE. 2009;4:e5515. doi: 10.1371/journal.pone.0005515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang K., Huang J., Xie W., Huang L., Zhong C., Chen Z. Beclin1 and HMGB1 Ameliorate the α-Synuclein-Mediated Autophagy Inhibition in PC12 Cells. Diagn. Pathol. 2016;11:15. doi: 10.1186/s13000-016-0459-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Martinez-Vicente M., Talloczy Z., Kaushik S., Massey A.C., Mazzulli J., Mosharov E.V., Hodara R., Fredenburg R., Wu D.C., Follenzi A., et al. Dopamine-Modified α-Synuclein Blocks Chaperone-Mediated Autophagy. J. Clin. Invest. 2008;118:777. doi: 10.1172/JCI32806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Qian Y., Hua S., Marla G., Emanuela C., Michael L., Shacka J.J., Zixu M. Regulation of Neuronal Survival Factor MEF2D by Chaperone-Mediated Autophagy. Science. 2009;323:124–127. doi: 10.1126/science.1166088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Usenovic M., Tresse E., Mazzulli J.R., Taylor J.P., Krainc D. Deficiency of ATP13A2 Leads to Lysosomal Dysfunction, α-Synuclein Accumulation, and Neurotoxicity. J. Neurosci. 2012;32:4240–4246. doi: 10.1523/JNEUROSCI.5575-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang B., Cai Z., Tao K., Zeng W., Lu F., Yang R., Feng D., Gao G., Yang Q. Essential Control of Mitochondrial Morphology and Function by Chaperone-Mediated Autophagy through Degradation of PARK7. Autophagy. 2016;12:1215–1228. doi: 10.1080/15548627.2016.1179401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Thomas K.J., McCoy M.K., Blackinton J., Beilina A., van der Brug M., Sandebring A., Miller D., Maric D., Cedazo-Minguez A., Cookson M.R. DJ-1 Acts in Parallel to the PINK1/Parkin Pathway to Control Mitochondrial Function and Autophagy. Hum. Mol. Genet. 2011;20:40–50. doi: 10.1093/hmg/ddq430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bae E.J., Yang N.Y., Lee C., Lee H.J., Kim S., Sardi S.P., Lee S.J. Loss of Glucocerebrosidase 1 Activity Causes Lysosomal Dysfunction and α-Synuclein Aggregation. Exp. Mol. Med. 2015;47:e153. doi: 10.1038/emm.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mazzulli J.R., Xu Y.H., Sun Y., Knight A.L., McLean P.J., Caldwell G.A., Sidransky E., Grabowski G.A., Krainc D. Gaucher Disease Glucocerebrosidase and α-Synuclein Form a Bidirectional Pathogenic Loop in Synucleinopathies. Cell. 2011;146:37–52. doi: 10.1016/j.cell.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fanciulli A., Stankovic I., Krismer F., Seppi K., Levin J., Wenning G.K. Multiple System Atrophy. Int. Rev. Neurobiol. 2019;149:137–192. doi: 10.1016/bs.irn.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 129.Wenning G.K., Stefanova N., Jellinger K.A., Poewe W., Schlossmacher M.G. Multiple System Atrophy: A Primary Oligodendrogliopathy. Ann. Neurol. 2008;64:239–246. doi: 10.1002/ana.21465. [DOI] [PubMed] [Google Scholar]
- 130.Asi Y.T., Simpson J.E., Heath P.R., Wharton S.B., Lees A.J., Revesz T., Houlden H., Holton J.L. Alpha-Synuclein MRNA Expression in Oligodendrocytes in MSA. Glia. 2014;62:964–970. doi: 10.1002/glia.22653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kaji S., Maki T., Kinoshita H., Uemura N., Ayaki T., Kawamoto Y., Furuta T., Urushitani M., Hasegawa M., Kinoshita Y., et al. Pathological Endogenous α-Synuclein Accumulation in Oligodendrocyte Precursor Cells Potentially Induces Inclusions in Multiple System Atrophy. Stem Cell Rep. 2018;10:356–365. doi: 10.1016/j.stemcr.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Djelloul M., Holmqvist S., Boza-Serrano A., Azevedo C., Yeung M.S., Goldwurm S., Frisén J., Deierborg T., Roybon L. Alpha-Synuclein Expression in the Oligodendrocyte Lineage: An In Vitro and In Vivo Study Using Rodent and Human Models. Stem Cell Rep. 2015;5:174–184. doi: 10.1016/j.stemcr.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Miller D.W., Johnson J.M., Solano S.M., Hollingsworth Z.R., Standaert D.G., Young A.B. Absence of α-Synuclein MRNA Expression in Normal and Multiple System Atrophy Oligodendroglia. J. Neural Transm. 2005;112:1613–1624. doi: 10.1007/s00702-005-0378-1. [DOI] [PubMed] [Google Scholar]
- 134.Reyes J.F., Rey N.L., Bousset L., Melki R., Brundin P., Angot E. Alpha-Synuclein Transfers from Neurons to Oligodendrocytes. Glia. 2014;62:387–398. doi: 10.1002/glia.22611. [DOI] [PubMed] [Google Scholar]
- 135.Danzer K.M., Kranich L.R., Ruf W.P., Cagsal-Getkin O., Winslow A.R., Zhu L., Vanderburg C.R., McLean P.J. Exosomal Cell-to-Cell Transmission of Alpha Synuclein Oligomers. Mol. Neurodegener. 2012;7:1–18. doi: 10.1186/1750-1326-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Fellner L., Buchinger E., Brueck D., Irschick R., Wenning G.K., Stefanova N. Limited Effects of Dysfunctional Macroautophagy on the Accumulation of Extracellularly Derived α-Synuclein in Oligodendroglia: Implications for MSA Pathogenesis. BMC Neurosci. 2018;19:32. doi: 10.1186/s12868-018-0431-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ettle B., Reiprich S., Deusser J., Schlachetzki J.C.M., Xiang W., Prots I., Masliah E., Winner B., Wegner M., Winkler J. Intracellular Alpha-Synuclein Affects Early Maturation of Primary Oligodendrocyte Progenitor Cells. Mol. Cell. Neurosci. 2014;62:68–78. doi: 10.1016/j.mcn.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pukaß K., Richter-Landsberg C. Oxidative Stress Promotes Uptake, Accumulation, and Oligomerization of Extracellular α-Synuclein in Oligodendrocytes. J. Mol. Neurosci. 2013;52:339–352. doi: 10.1007/s12031-013-0154-x. [DOI] [PubMed] [Google Scholar]
- 139.Pukaß K., Goldbaum O., Richter-Landsberg C. Mitochondrial Impairment and Oxidative Stress Compromise Autophagosomal Degradation of α-Synuclein in Oligodendroglial Cells. J. Neurochem. 2015;135:194–205. doi: 10.1111/jnc.13256. [DOI] [PubMed] [Google Scholar]
- 140.Rockenstein E., Ubhi K., Inglis C., Mante M., Patrick C., Adame A., Masliah E. Neuronal to Oligodendroglial Alpha-Synuclein Redistribution in a Double Transgenic Model of Multiple System Atrophy. Neuroreport. 2012;23:259–264. doi: 10.1097/WNR.0b013e3283509842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mavroeidi P., Arvanitaki F., Karakitsou A.K., Vetsi M., Kloukina I., Zweckstetter M., Giller K., Becker S., Sorrentino Z.A., Giasson B.I., et al. Endogenous Oligodendroglial Alpha-Synuclein and TPPP/P25α Orchestrate Alpha-Synuclein Pathology in Experimental Multiple System Atrophy Models. Acta Neuropathol. 2019;138:415–441. doi: 10.1007/s00401-019-02014-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Konno M., Hasegawa T., Baba T., Miura E., Sugeno N., Kikuchi A., Fiesel F.C., Sasaki T., Aoki M., Itoyama Y., et al. Suppression of Dynamin GTPase Decreases α-Synuclein Uptake by Neuronal and Oligodendroglial Cells: A Potent Therapeutic Target for Synucleinopathy. Mol. Neurodegener. 2012;7:38. doi: 10.1186/1750-1326-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kisos H., Pukaß K., Ben-Hur T., Richter-Landsberg C., Sharon R. Increased Neuronal α-Synuclein Pathology Associates with Its Accumulation in Oligodendrocytes in Mice Modeling α-Synucleinopathies. PLoS ONE. 2012;7:46817. doi: 10.1371/journal.pone.0046817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Woerman A.L., Watts J.C., Aoyagi A., Giles K., Middleton L.T., Prusiner S.B. A-Synuclein: Multiple System Atrophy Prions. Cold Spring Harb. Perspect. Med. 2018;8:a024588. doi: 10.1101/cshperspect.a024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Prusiner S.B., Woerman A.L., Mordes D.A., Watts J.C., Rampersaud R., Berry D.B., Patel S., Oehler A., Lowe J.K., Kravitz S.N., et al. Evidence for α-Synuclein Prions Causing Multiple System Atrophy in Humans with Parkinsonism. Proc. Natl. Acad. Sci. USA. 2015;112:E5308–E5317. doi: 10.1073/pnas.1514475112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Woerman A.L., Oehler A., Kazmi S.A., Lee J., Halliday G.M., Middleton L.T., Gentleman S.M., Mordes D.A., Spina S., Grinberg L.T., et al. Multiple System Atrophy Prions Retain Strain Specificity after Serial Propagation in Two Different Tg(SNCA*A53T) Mouse Lines. Acta Neuropathol. 2019;137:437. doi: 10.1007/s00401-019-01959-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Tamgüney G., Korczyn A.D. A Critical Review of the Prion Hypothesis of Human Synucleinopathies. Cell Tissue Res. 2017;373:213–220. doi: 10.1007/s00441-017-2712-y. [DOI] [PubMed] [Google Scholar]
- 148.Vascellari S., Manzin A. Parkinson’s Disease: A Prionopathy? Int. J. Mol. Sci. 2021;22:8022. doi: 10.3390/ijms22158022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Schwarz L., Goldbaum O., Bergmann M., Probst-Cousin S., Richter-Landsberg C. Involvement of Macroautophagy in Multiple System Atrophy and Protein Aggregate Formation in Oligodendrocytes. J. Mol. Neurosci. 2012;47:256–266. doi: 10.1007/s12031-012-9733-5. [DOI] [PubMed] [Google Scholar]
- 150.Masui K., Nakata Y., Fujii N., Iwaki T. Extensive Distribution of Glial Cytoplasmic Inclusions in an Autopsied Case of Multiple System Atrophy with a Prolonged 18-Year Clinical Course. Neuropathology. 2012;32:69–76. doi: 10.1111/j.1440-1789.2011.01222.x. [DOI] [PubMed] [Google Scholar]
- 151.Moreau K., Luo S., Rubinsztein D.C. Cytoprotective Roles for Autophagy. Curr. Opin. Cell Biol. 2010;22:206. doi: 10.1016/j.ceb.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Valera E., Spencer B., Mott J., Trejo M., Adame A., Mante M., Rockenstein E., Troncoso J.C., Beach T.G., Masliah E., et al. MicroRNA-101 Modulates Autophagy and Oligodendroglial Alpha-Synuclein Accumulation in Multiple System Atrophy. Front. Mol. Neurosci. 2017;10:329. doi: 10.3389/fnmol.2017.00329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takahashi K., Yamanaka S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 154.Compagnoni G.M., Kleiner G., Samarani M., Aureli M., Faustini G., Bellucci A., Ronchi D., Bordoni A., Garbellini M., Salani S., et al. Mitochondrial Dysregulation and Impaired Autophagy in IPSC-Derived Dopaminergic Neurons of Multiple System Atrophy. Stem Cell Rep. 2018;11:1185–1198. doi: 10.1016/j.stemcr.2018.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kahle P.J., Neumann M., Ozmen L., Müller V., Jacobsen H., Spooren W., Fuss B., Mallon B., Macklin W.B., Fujiwara H., et al. Hyperphosphorylation and Insolubility of α-Synuclein in Transgenic Mouse Oligodendrocytes. EMBO Rep. 2002;3:583. doi: 10.1093/embo-reports/kvf109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Stefanova N., Kaufmann W.A., Humpel C., Poewe W., Wenning G.K. Systemic Proteasome Inhibition Triggers Neurodegeneration in a Transgenic Mouse Model Expressing Human α-Synuclein under Oligodendrocyte Promoter: Implications for Multiple System Atrophy. Acta Neuropathol. 2012;124:51. doi: 10.1007/s00401-012-0977-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Emadi S., Kasturirangan S., Wang M.S., Schulz P., Sierks M.R. Detecting Morphologically Distinct Oligomeric Forms of α-Synuclein. J. Biol. Chem. 2009;284:11048–11058. doi: 10.1074/jbc.M806559200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xin W., Emadi S., Williams S., Liu Q., Schulz P., He P., Alam N.B., Wu J., Sierks M.R. Toxic Oligomeric Alpha-Synuclein Variants Present in Human Parkinson’s Disease Brains Are Differentially Generated in Mammalian Cell Models. Biomolecules. 2015;5:1634–1651. doi: 10.3390/biom5031634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Peelaerts W., Bousset L., Van der Perren A., Moskalyuk A., Pulizzi R., Giugliano M., Van den Haute C., Melki R., Baekelandt V. α-Synuclein Strains Cause Distinct Synucleinopathies after Local and Systemic Administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.