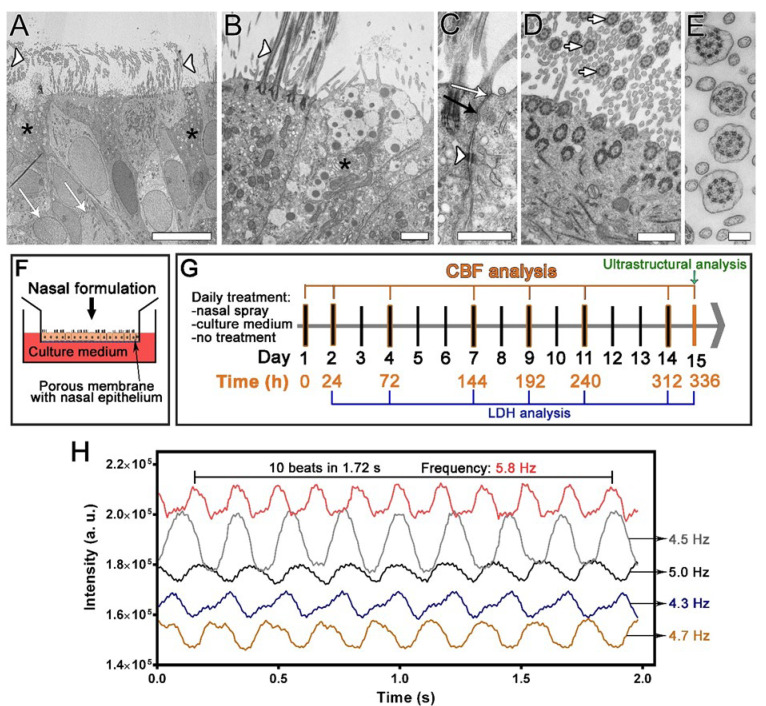
Figure 1.
The design of the repeated exposure study utilising the nasal MucilAir™ in vitro models and high-speed digital phase-contrast microscopy imaging. (A–E) Ultrastructural properties of the nasal in vitro model. (A,B) A pseudostratified epithelium with ciliated cells (A,B, white arrowheads indicate cilia), goblet cells (A,B, black asterisks), and basal cells (A, white arrows) is formed. (C) Adjacent cells are firmly connected by tight junctions (white arrow), adherent junctions (black arrow), and desmosomes (white arrowhead). (D,E) Characteristic axonemal structure with 9 × 2 + 2 arrangement of microtubules is viewed in cross section of the cilia (white arrows, D,E). Bars, 10 µm (A), 1 µm (B), 600 nm (C,D), 200 nm (E). (F) Nasal spray or culture medium was applied to the apical surface of the nasal in vitro models for 30 min daily and then washed off. (G) Cells were treated daily for 14 days, and the ciliary beat frequency (CBF) was analysed before the treatments (0 h) and at the indicated times. Ultrastructural analysis was performed at the end of the study. Lactate dehydrogenase (LDH) cytotoxicity assays were performed at the indicated times. (H) CBF measurements were performed by analysing the time-dependent greyscale intensity variations resulting from the repetitive cilia beating. Examples of the greyscale waveforms are shown, depicting the change in the grey intensity of the image at 5 different ROI as a function of time. The beat period was determined as the number of beats per unit time, from which the CBF was calculated (one divided by the beat period) and expressed in Hz.